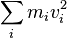Conservation of energy facts for kids
In physics and chemistry, the law of conservation of energy states that the total energy of an isolated system remains constant; it is said to be conserved over time. This law, first proposed and tested by Émilie du Châtelet, means that energy can neither be created nor destroyed; rather, it can only be transformed or transferred from one form to another. For instance, chemical energy is converted to kinetic energy when a stick of dynamite explodes. If one adds up all forms of energy that were released in the explosion, such as the kinetic energy and potential energy of the pieces, as well as heat and sound, one will get the exact decrease of chemical energy in the combustion of the dynamite.
Classically, conservation of energy was distinct from conservation of mass. However, special relativity showed that mass is related to energy and vice versa by E = mc2, and science now takes the view that mass-energy as a whole is conserved. Theoretically, this implies that any object with mass can itself be converted to pure energy, and vice versa. However this is believed to be possible only under the most extreme of physical conditions, such as likely existed in the universe very shortly after the Big Bang or when black holes emit Hawking radiation.
A consequence of the law of conservation of energy is that a perpetual motion machine of the first kind cannot exist, that is to say, no system without an external energy supply can deliver an unlimited amount of energy to its surroundings.
History
Ancient philosophers as far back as Thales of Miletus had the idea that there is some underlying substance of which everything is made. But that is not the same as our concept of "mass-energy" today (for example, Thales thought the underlying substance was water). In 1638, Galileo published his analysis of several situations. This included the famous "interrupted pendulum". This can be described (in modernized language) as conservatively converting potential energy to kinetic energy and back again. However, Galileo did not explain the process in modern terms and had not understood the modern concept either. The German Gottfried Wilhelm Leibniz during 1676-1689 attempted a mathematical formulation of the kind of energy which is connected with motion (kinetic energy). Leibniz noticed that in many mechanical systems (of several masses, mi each with velocity vi ),
was conserved so long as the masses did not interact. He called this quantity the vis viva or living force of the system. The principle represents an accurate statement of the approximate conservation of kinetic energy in situations where there is no friction.
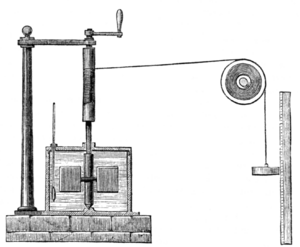
Meanwhile, in 1843 James Prescott Joule independently discovered the mechanical equivalent in a series of experiments. In the most famous, now called the "Joule apparatus", a descending weight attached to a string caused a paddle immersed in water to rotate. He showed that the gravitational potential energy lost by the weight in descending was approximately equal to the thermal energy (heat) gained by the water by friction with the paddle.
Over the period 1840-1843, similar work was carried out by engineer Ludwig A. Colding though it was little-known outside his native Denmark.
Images for kids
-
Emmy Noether (1882-1935) was an influential mathematician known for her groundbreaking contributions to abstract algebra and theoretical physics.
See also
 In Spanish: Conservación de la energía para niños
In Spanish: Conservación de la energía para niños