States of matter facts for kids
There are four common states of matter (or phases) in the universe: solid, liquid, gas, and plasma. The state of matter affects a substance's properties, such as density, viscosity (how well it flows), malleability (how easy it is to bend), and conductivity.
Contents
Common states of matter
Solids
In a solid, the positions of atoms are fixed relative to each other over long time. That is due to the cohesion or "friction" between molecules. This cohesion is provided by metallic, covalent or ionic bonds. Only solids can be pushed on by a force without changing shape, which means that they can be resistant to deformation. Solids also tend to be strong enough to hold their own shape in a container. Solids are generally denser than liquids. Solid becoming a gas is sublimation.
Liquids
In a liquid, molecules are attracted to other molecules strong enough to keep molecules in contact, but not strong enough to fix a particular structure. The molecules can continually move with respect to each other. This means that liquids can flow smoothly, but not as smoothly as gases. Liquids will tend to take the shape of a container that they are in. Liquids are generally less dense than solids, but denser than gases.
Gases
In a gas, the chemical bonds are not strong enough to hold atoms or molecules together, and from this a gas is a collection of independent, unbonded molecules which interact mainly by collision. Gases tend to take the shape of their container, and are less dense than both solids and liquids. Gases have smaller forces of attraction than solids and liquids. Gas becoming a solid is deposition
Plasmas
Plasmas are gases that have so much energy that electrons of an atom cannot stay in orbit around one atomic nucleus. The atomic ions and free electrons mix around like a hot soup.
Because the positive and negative charged particles are not stuck together, plasma is a good conductor of electricity. For example, air is not good at conducting electricity. However, in a bolt of lightning, the atoms in air get so much energy that they no longer can hold on to their electrons, and become a plasma for a brief time. Then an electric current is able to flow through the plasma, making the lightning.
Plasma is the most common state of matter in the universe. Both stars and the interstellar medium are mostly made of plasma.
Phase changes
Phases of matter can be changed by a number of things. This includes pressure and temperature.
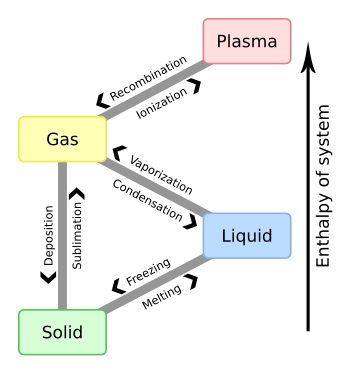
When a solid becomes a liquid, it is called melting. When a solid becomes a gas, it is called sublimation. When a liquid becomes a gas, it is called evaporation. When a gas becomes a liquid, it is called condensation. When a liquid becomes a solid, it is called freezing. The freezing point and the melting point are said to be the same, because any increase in temperature will cause it to melt and any drop in temperature will cause it to freeze. This is also the reason that the vaporizing and condensation point are the same.
Other states
Many other states of matter can exist under special conditions, including strange matter, superfluids, and supersolids, and possibly string-net liquids. Scientist work on new experiments at higher temperatures and energy levels than have ever been made. They also work on experiments at very low temperatures. Such experiments help scientists learn more about phases of matter.
Quark-gluon plasmas
Quark-gluon plasmas are a relatively newly discovered phase of matter that happen at about 2 trillion Kelvin. Scientists believe that protons and neutrons are held together by tiny things called quarks (which are "glued" together by things called "gluons"). At an incredibly high temperature only achievable by the Large Hadron Collider at CERN, quarks and gluons begin to separate into a new state of matter. Little is known about quark-gluon plasmas because of the huge amount of energy needed to make them.
Bose-Einstein condensates
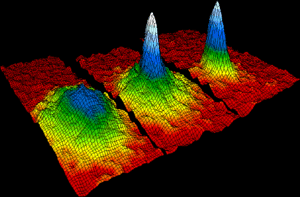
Bose-Einstein condensates and fermionic condensates are phases of matter that apply to particles called bosons and fermions, respectively. (More than one boson can exist in the same spot at the same time. Only one fermion can exist in the same spot at the same time). Bose-Einstein condensates and fermionic condensates occur at incredibly low temperatures (about 4° Kelvin, which is the same as -452° Fahrenheit). Little is known about either state because of the sheer amount of energy needed to be taken away to create them. Inside of them, all of the particles begin to act like one big quantum state. That is, they have near-zero electrical resistance, and have almost no friction.
Images for kids
See also
 In Spanish: Estado de agregación de la materia para niños
In Spanish: Estado de agregación de la materia para niños