Image: Nylon-3D-h bond
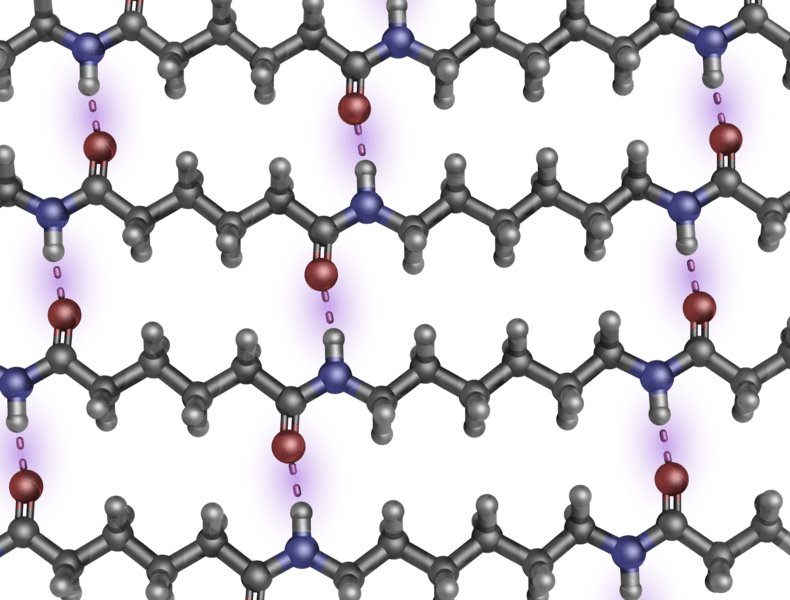
Description: Hydrogen bonding in nylon 6-6. The nylon fibers are relatively resistant to damage because their long-chain molecules have stronger inter-molecular forces than the Van der Waals bonds between polyethylene chains. Indeed, each N—H group in a nylon chain can hydrogen-bond to the O of a C=O group in a neighboring chain, as shown above. Therefore, the chains cannot slide past one another easily ChemPRIME Staff (2010), Condensation Polymers, chemeddl.org.
Title: Nylon-3D-h bond
Credit: Own work
Author: GYassineMrabetTalk✉ This image was created with PyMOL
Permission: This file is licensed under the Creative Commons Attribution 3.0 Unported license. You are free: to share – to copy, distribute and transmit the work to remix – to adapt the work Under the following conditions: attribution – You must attribute the work in the manner specified by the author or licensor (but not in any way that suggests that they endorse you or your use of the work). http://creativecommons.org/licenses/by/3.0 CC BY 3.0 Creative Commons Attribution 3.0 truetrue
Usage Terms: Creative Commons Attribution 3.0
License: CC BY 3.0
License Link: http://creativecommons.org/licenses/by/3.0
Attribution Required?: Yes
Image usage
The following page links to this image:

