Calorimeter facts for kids
A calorimeter is a special tool. It helps scientists and engineers measure heat. It can tell us how much heat energy is inside something. Or, it can measure how much heat is released during a chemical reaction. For example, it's used to find out how much heat food makes when it burns. This helps us understand its energy content.
Contents
What is a Calorimeter?
A calorimeter is a device that measures the amount of heat involved in a process. This process could be a chemical reaction, a physical change, or even a biological event. The word "calorimeter" comes from the Latin word "calor," which means heat.
Scientists use calorimeters to study how much energy is stored in different substances. They also use them to understand how much energy is released or absorbed during reactions. This is important in many fields, like chemistry, biology, and even food science.
How Does a Calorimeter Work?
Most calorimeters work by measuring temperature changes. Imagine you have a reaction happening inside a special container. This container is surrounded by water. As the reaction gives off or takes in heat, the temperature of the water changes.
By knowing the mass of the water and how much its temperature changed, scientists can calculate the amount of heat. This is because water absorbs or releases heat in a very predictable way. The calorimeter is usually insulated. This means it's designed to stop heat from escaping or entering from the outside. This makes the measurements more accurate.
Measuring Heat Energy
The main idea behind a calorimeter is simple. If a reaction releases heat, the temperature inside the calorimeter goes up. If a reaction absorbs heat, the temperature goes down. The calorimeter measures this temperature change very carefully.
Scientists use a formula to turn the temperature change into a heat value. This formula considers the specific heat capacity of the materials inside. Specific heat capacity tells us how much energy is needed to raise the temperature of a substance by a certain amount.
Types of Calorimeters
There are different kinds of calorimeters, each designed for specific uses.
Bomb Calorimeter
A bomb calorimeter is a strong, sealed container. It is often used to measure the heat released when something burns. This is called the heat of combustion. Food scientists use bomb calorimeters to find out the calorie content of food.
Inside the "bomb," a small sample of the substance is placed. It is then burned completely in an oxygen-rich environment. The heat released warms the water surrounding the bomb. By measuring the water's temperature change, the heat of combustion is calculated.
Ice Calorimeter
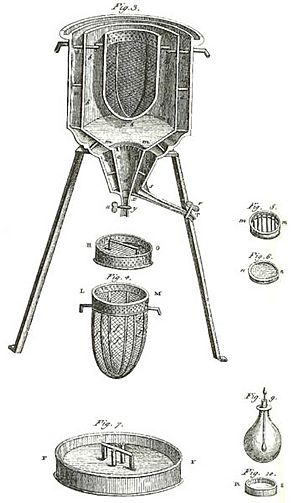
An ice calorimeter works a bit differently. Instead of measuring temperature change, it measures how much ice melts. When a reaction releases heat inside an ice calorimeter, it melts some of the surrounding ice.
The amount of melted ice tells scientists how much heat was released. This type of calorimeter was one of the first ever invented. Antoine Lavoisier and Pierre-Simon Laplace used one in the late 1700s.
Solution Calorimeter
A solution calorimeter is used to measure heat changes when substances dissolve or react in a liquid. It's often used to study how much heat is released or absorbed when chemicals mix in a solution.
Why Are Calorimeters Important?
Calorimeters are very important tools in science and industry.
- Food Science: They help determine the calorie content of food. This is how we know how much energy different foods provide.
- Chemistry: They are used to study chemical reactions. This helps scientists understand how much energy is needed or released during different processes.
- Material Science: They help test new materials. For example, they can measure how much heat a new building material can hold.
- Safety: In industry, understanding heat release is crucial for safety. It helps prevent dangerous temperature buildups in factories.
History of Calorimeters
The idea of measuring heat accurately goes back a long way.
Early Discoveries
In the 1700s, scientists started to understand heat better. Joseph Black, a Scottish chemist, discovered the idea of "latent heat." This is the hidden heat absorbed or released when a substance changes its state, like ice melting into water.
Lavoisier and Laplace
Building on Black's work, French scientists Antoine Lavoisier and Pierre-Simon Laplace created the first ice calorimeter in 1782-83. They used it to measure the heat produced by chemical reactions and even by living things. Their experiments were a big step in starting the field of thermochemistry. Thermochemistry is the study of heat changes during chemical reactions.
Their work showed that heat is a measurable quantity. It helped scientists understand energy changes in a new way. From these early designs, calorimeters have become much more advanced. Today, they are precise instruments used in many scientific fields.
Images for kids
See also
 In Spanish: Calorímetro para niños
In Spanish: Calorímetro para niños