Coordination geometry facts for kids
Coordination geometry is a cool way to understand how atoms are arranged in molecules. Think of it like building blocks! When atoms join together to form a molecule, they don't just stick randomly. They settle into specific shapes, like a cube, a line, a triangle, or a pyramid. This arrangement of atoms around a central atom is what we call coordination geometry. It's super important in chemistry because the shape of a molecule affects how it behaves and what it can do!
Contents
What is Coordination Geometry?
Coordination geometry describes the pattern of atoms surrounding a central atom in a molecule or a crystal. Imagine you have a main atom, and other atoms are connected to it. The way these connected atoms are positioned in space around the central one creates a specific geometric shape. This shape is not just random; it's determined by the number of atoms connected and the way their electrons interact.
Why Molecular Shapes Matter
The shape of a molecule is a big deal in chemistry. It affects almost everything about the molecule, like:
- How it reacts: A molecule's shape can decide if it fits with another molecule, like a key fitting into a lock.
- Its properties: Shape can influence if a substance is a gas, liquid, or solid, or if it dissolves in water.
- Biological functions: In your body, proteins and DNA have very specific shapes that allow them to do their jobs, like carrying oxygen or storing genetic information.
Understanding these shapes helps scientists design new medicines, create new materials, and even understand how life works!
Common Molecular Shapes
Molecules can take on many different shapes, depending on how many atoms are connected to the central atom and how the electrons are arranged. Here are some of the most common shapes you'll find:
Linear Shape
A linear shape is the simplest. It means all the atoms are arranged in a straight line.
- Imagine three atoms, with one in the middle and one on each side, forming a 180-degree angle.
- A good example is carbon dioxide (CO₂), the gas we breathe out. The carbon atom is in the middle, and the two oxygen atoms are on opposite sides, making a straight line.
Trigonal Planar Shape
In a trigonal planar shape, a central atom is connected to three other atoms, and all four atoms lie in the same flat plane.
- Think of a flat triangle. The central atom is at the very center, and the three other atoms are at the corners of the triangle.
- The angles between the atoms are usually 120 degrees.
- An example is boron trifluoride (BF₃), where a boron atom is in the middle of three fluorine atoms.
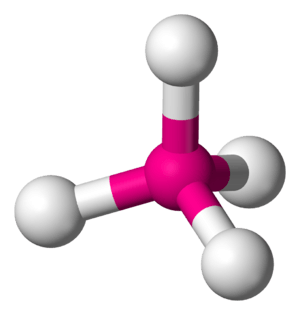
Tetrahedral Shape
The tetrahedral shape is very common, especially in organic chemistry.
- Here, a central atom is connected to four other atoms. These four atoms are positioned around the central one to form a pyramid with a triangular base.
- The angles between the atoms are about 109.5 degrees.
- Methane (CH₄), the main component of natural gas, has a tetrahedral shape. The carbon atom is in the center, and four hydrogen atoms are at the corners.
Trigonal Bipyramidal Shape
A trigonal bipyramidal shape involves a central atom connected to five other atoms.
- This shape looks like two pyramids joined at their bases. The base is a triangle (trigonal), and there are two atoms sticking out from the top and bottom (bipyramidal).
- The angles can be 90 degrees (for the atoms pointing up/down) or 120 degrees (for the atoms in the triangular plane).
- An example is phosphorus pentachloride (PCl₅).
Octahedral Shape
The octahedral shape is when a central atom is connected to six other atoms.
- This shape looks like two square-based pyramids joined at their bases.
- All the angles between the atoms are 90 degrees.
- A common example is sulfur hexafluoride (SF₆).
How Shapes Are Predicted
Scientists use something called the VSEPR theory (Valence Shell Electron Pair Repulsion theory) to predict these shapes. It sounds complicated, but the basic idea is simple:
- Electrons around an atom repel each other (they push away from each other) because they all have a negative charge.
- To minimize this repulsion, the electron pairs (and the atoms they connect to) arrange themselves as far apart as possible in space.
- This "farthest apart" arrangement is what gives molecules their specific coordination geometry!
So, the shape of a molecule isn't just random; it's a result of the tiny electrons trying to get as much space as possible from each other.
 | Delilah Pierce |
 | Gordon Parks |
 | Augusta Savage |
 | Charles Ethan Porter |