Differential scanning calorimeter facts for kids
Differential scanning calorimetry (DSC) is a special tool used in science to study how materials change when they are heated or cooled. It helps scientists learn about the heat changes that happen inside substances. This is important for understanding many things, like how new materials are made, how medicines are pure, and even the quality of food.
DSC was first created in 1962 by E.S. Watson and M.J. O’Neil. It became available to buy in 1963. It is very fast and easy to use, giving quick information about how materials react to heat.
Contents
How DSC Works
There are two main types of DSC machines. One is called heat flux DSC, which keeps the heat flowing into the system at a steady rate. The other is power-compensated DSC, which keeps the power supplied to the machine constant.
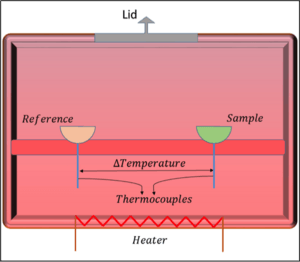
In general, a DSC machine works by measuring the difference in temperature between two places:
- The sample holder holds the material you want to study.
- The reference holder usually stays empty.
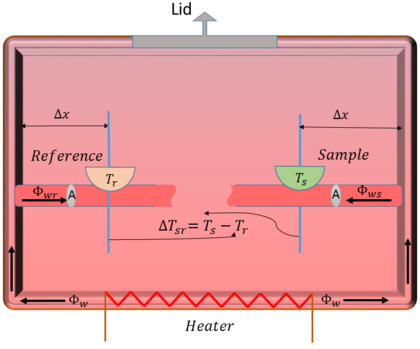
Both holders sit on a support that is connected to the machine's walls. A heating resistor warms up the walls, creating the right amount of heat inside. A thermocouple is a device that measures the temperature of both the sample and the reference. This temperature information is then used for the analysis. The heat from the heating resistor flows into both the sample and reference areas.
Understanding DSC Results
To understand how DSC works, imagine a simple model. In this model:
- Heat flows at a steady rate.
- The sample and reference do not affect each other.
- We mainly look at how much heat the sample and reference can hold.
- The system is closed, meaning no heat escapes to the outside.
A basic rule called Fourier's law helps explain how heat moves through materials. It says that heat energy moves from hotter areas to colder areas. In DSC, a computer adds heat to both the sample and reference holders.
When the sample holder has a substance and the reference is empty, the temperature of the sample holder can change:
- If the substance needs heat to change (like ice melting), its temperature goes down. This is called an endothermic process.
- If the substance releases heat when it changes (like something burning), its temperature goes up. This is called an exothermic process.
DSC measures these temperature changes. It then figures out how much heat was absorbed or released. The main result of a DSC experiment is a graph called a DSC curve. This graph shows how the heat flow changes as the temperature changes. By looking at these curves, scientists can learn about how much heat is involved in different changes, like melting, freezing, or chemical reactions.
What DSC Is Used For
Proteins Changing Shape
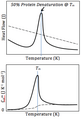
One important use of DSC is studying proteins. Proteins are tiny building blocks in our bodies. When heated, proteins can change their shape, a process called denaturation. DSC helps scientists find the temperature range where proteins start to change. It can also measure how much heat proteins can hold. Denatured proteins hold more heat, so DSC can show how much a protein has changed its shape over time.
Checking Fats and Oils
DSC is also very useful for checking the quality of food, especially fats and oils. Sometimes, cheaper or harmful ingredients are mixed into expensive food products. This is called adulteration. DSC can analyze how fats and oils behave when they are cooled and heated.
- The cooling process shows how they crystallize (turn solid).
- The heating process shows how they melt.
If a fat or oil has been mixed with other things, its DSC curves will look different. New peaks might appear, or existing peaks might change. By studying these changes, scientists can tell if a food product has been adulterated.
Testing Drug Purity
DSC is also very popular for checking how pure a drug is. It only needs a very small amount of the drug (about 1-2 milligrams) and gives results quickly. If a drug has impurities (other substances mixed in), its melting temperature will usually decrease.
The melting temperature of a drug also shows how stable it is. A higher melting temperature means the drug is more stable and will last longer. DSC allows scientists to quickly check this temperature. This makes it much easier and faster to control the quality of medicines.
Images for kids
See also
 In Spanish: Calorimetría diferencial de barrido para niños
In Spanish: Calorimetría diferencial de barrido para niños
 | Laphonza Butler |
 | Daisy Bates |
 | Elizabeth Piper Ensley |