Emulsion facts for kids
An emulsion is a special kind of mixture where two liquids don't really mix together. Think about oil and water: no matter how much you shake them, the oil always separates and floats on top of the water. This happens because these liquids are "immiscible," meaning they can't dissolve in each other.
When you shake oil and water, the oil breaks into tiny droplets that spread out in the water. But if you leave them alone, these tiny droplets will eventually join back together, and the oil will separate from the water again. To stop this separation and keep the liquids mixed, we need something called an emulsifier.
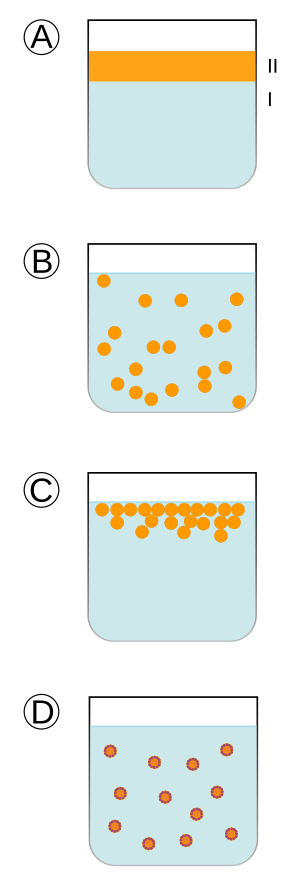
* A: The two liquids are separated, one is on top, the other at the bottom.
* B: The second liquid is spread out in the first.
* C: An unstable emulsion separates (which will lead to picture A).
* D: A Surfactant (emulsifier) positions itself between the two liquids.
What is an Emulsion?
An emulsion is a mixture of two or more liquids that usually don't mix. Imagine trying to mix oil and vinegar for salad dressing. If you just pour them together, they stay separate. But if you shake them really hard, the oil breaks into tiny drops that spread through the vinegar. This temporary mixture is an emulsion. Over time, the oil droplets will gather again, and the liquids will separate.
Why Liquids Don't Mix
Some liquids, like water, are "polar." This means their molecules have a slight positive end and a slight negative end, like tiny magnets. Other liquids, like oil, are "non-polar." Their molecules don't have these charged ends. Polar liquids like to stick with other polar liquids, and non-polar liquids like to stick with other non-polar liquids. They don't like to mix with each other. This is why oil and water stay separate.
How Emulsifiers Help
To make an emulsion stable, so the liquids stay mixed for a long time, we use an emulsifier. An emulsifier is a special molecule that acts like a bridge between the two liquids. It has two different parts:
- Water-loving part: This part is called "hydrophilic" (hydro means water, philic means loving). It likes to connect with water molecules.
- Oil-loving part: This part is called "hydrophobic" (hydro means water, phobic means fearing). It doesn't like water, but it loves to connect with oil molecules.
When you add an emulsifier to oil and water, the emulsifier molecules surround the tiny oil droplets. The oil-loving part of the emulsifier sticks to the oil, and the water-loving part faces outwards into the water. This creates a protective layer around each oil droplet, stopping them from joining back together. This way, the oil stays spread out in the water, creating a stable emulsion.
Many everyday products are emulsions. For example, milk is an emulsion of tiny fat droplets in water, with proteins acting as emulsifiers. Mayonnaise is another common emulsion, made from oil and vinegar, with egg yolk acting as the emulsifier.
Related pages
See also
 In Spanish: Emulsión para niños
In Spanish: Emulsión para niños

