Hydrogen sulfide facts for kids
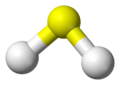
Hydrogen sulfide (also spelled hydrogen sulphide in British English) is a chemical compound. It has the chemical formula H2S. This means it is made of two hydrogen atoms and one sulfur atom. It is a colorless gas, which means you cannot see it.
Hydrogen sulfide is known for its strong, unpleasant smell. It smells just like rotten eggs! It is also a very toxic (poisonous) and flammable (can easily catch fire) gas.
This gas often forms when bacteria break down organic matter (like dead plants and animals) in places where there is no oxygen. You can find it in swamps and sewers. It is also present in volcanic gases, natural gas, and some well waters. Many people think this smell comes from sulfur, but sulfur itself does not actually smell.
Hydrogen sulfide has other names too. Some of these are sulfane, sulfur hydride, sour gas, and sewer gas. The International Union of Pure and Applied Chemistry (IUPAC) accepts "hydrogen sulfide" and "sulfane" as correct names.
Contents
Where is Hydrogen Sulfide Found?
You can find small amounts of hydrogen sulfide in crude oil. Some natural gas, called "sour natural gas," can have a lot of it, up to 28%. This sour gas must be cleaned before it can be sent through long pipelines. Pipelines usually allow only tiny amounts of hydrogen sulfide.
Volcanoes and hot springs also release some H2S. It is likely made there when water reacts with sulfide minerals in the ground. In clean air, the normal amount of hydrogen sulfide is very, very low. It is about 0.0001 to 0.0002 parts per million (ppm).
Why is Hydrogen Sulfide Dangerous?
Hydrogen sulfide is a very toxic and flammable gas. Because it is heavier than air, it tends to collect in low, poorly ventilated areas. This means it can build up in basements, trenches, or sewers.
How Does it Affect the Body?
Hydrogen sulfide is a broad-spectrum poison. This means it can harm many different systems in your body. The nervous system, which includes your brain and nerves, is affected the most. The danger of H2S is similar to that of hydrogen cyanide, another very poisonous gas. Even a small amount can be harmful.
If you breathe in too much hydrogen sulfide, it can cause serious health problems. It can even be deadly. This is why it is very important to be careful around places where this gas might be present.
Images for kids
See also
 In Spanish: Ácido sulfhídrico para niños
In Spanish: Ácido sulfhídrico para niños