Latent heat facts for kids
Latent heat is the amount of hidden energy needed to change a substance's state. This means turning a solid into a liquid, a liquid into a gas, or the other way around. For example, it's the energy needed for water to boil into steam, or for water to freeze into ice. It's called "latent" because this energy doesn't make the temperature go up or down on a thermometer while the change is happening.
Contents
Latent Heat: The Hidden Energy in State Changes
Latent heat is a special kind of energy. When a substance changes from one state to another, like from ice (solid) to water (liquid), it needs energy. But this energy doesn't make the substance hotter. Instead, it's used to break or form the bonds between the particles of the substance.
Why is it "Latent"? Uncovering the Secret
The word "latent" means hidden or not obvious. Imagine you are boiling water. You add heat, and the water gets hotter until it reaches 100 degrees Celsius (212 degrees Fahrenheit). At this point, even if you keep adding heat, the water's temperature won't go above 100°C. Instead, the water starts turning into steam. The extra heat you're adding is the latent heat. It's "hidden" because it's not making the thermometer rise; it's busy changing the water into steam.
Examples of Latent Heat in Everyday Life
Latent heat is all around us!
- Boiling water: When water boils, it absorbs a lot of latent heat to turn into steam. This is why steam burns are so dangerous – the steam releases this hidden heat when it condenses back into liquid water on your skin.
- Melting ice: When ice melts, it absorbs latent heat from its surroundings. This is why ice is great for cooling drinks. It absorbs heat from the drink without its own temperature changing until all the ice has melted.
- Sweating: Your body uses latent heat to cool down. When you sweat, the liquid sweat on your skin absorbs latent heat from your body to evaporate into a gas. This takes heat away from you, making you feel cooler.
Types of Latent Heat: Fusion and Vaporization
There are two main types of latent heat:
- Latent Heat of Fusion: This is the energy needed to change a substance from a solid to a liquid (melting) or from a liquid to a solid (freezing). For water, a lot of energy is needed to melt ice into water.
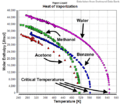
- Latent Heat of Vaporization: This is the energy needed to change a substance from a liquid to a gas (boiling or evaporation) or from a gas to a liquid (condensation). It takes even more energy to turn water into steam than it does to melt ice.
How Latent Heat Affects Our World
Latent heat plays a huge role in weather and climate:
- Clouds and rain: When water vapor in the air cools, it releases its latent heat of vaporization as it condenses into tiny water droplets, forming clouds. When these droplets get big enough, they fall as rain.
- Hurricanes: The massive energy in hurricanes comes partly from the latent heat released when huge amounts of water vapor condense into rain. This released heat fuels the storm.
- Cooling systems: Refrigerators and air conditioners work by using substances that absorb and release latent heat as they change state, moving heat from inside to outside.
Images for kids
See also
 In Spanish: Calor latente para niños
In Spanish: Calor latente para niños