VSEPR theory facts for kids
The way a molecule or ion looks is decided by how the tiny electron pairs around its central atom push each other away. Think of it like magnets repelling each other!
Contents
Understanding Molecule Shapes
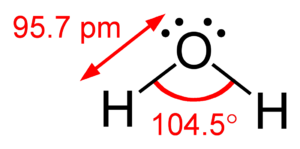
Molecules are tiny groups of atoms held together. Their shape is super important because it affects how they react with other molecules. The Valence Shell Electron-Pair Repulsion Theory, often called VSEPR (say "ves-per"), helps us predict these shapes.
What is VSEPR Theory?
VSEPR theory is a simple idea that helps scientists figure out the 3D shape of molecules. It's all about the electrons around the central atom in a molecule. These electrons are always moving and they try to get as far away from each other as possible. This "pushing away" is called repulsion.
How Electrons Repel Each Other
Imagine you have a bunch of balloons tied together at one point. They will naturally spread out to give each balloon as much space as possible. Electrons do the same thing!
- Only the electron pairs around the main atom in a molecule are important for its shape.
- These pairs of electrons push each other away. They move to positions where they are furthest apart. This creates the molecule's unique shape.
Types of Electron Pairs
Not all electron pairs are the same. There are two main types:
- Bonding pairs: These are electrons that are shared between two atoms, forming a chemical bond.
- Lone pairs: These are electrons that belong to one atom but are not shared with another atom in a bond. They are "lonely" and not part of a bond.
Lone pairs take up more space than bonding pairs. This means they push harder!
- Two lone pairs push each other away the most. (Lone pair-lone pair repulsion is strongest).
- A lone pair and a bonding pair push each other away less strongly.
- Two bonding pairs push each other away the least. (Bonding pair-bonding pair repulsion is weakest).
How Electron Pairs Affect Shape
The actual shape of a molecule is determined by where the atoms are located around the central atom. However, the lone pairs of electrons, even though they aren't atoms, still influence the shape by pushing the bonding pairs around.
- Whether a bond is a single, double, or triple bond, it counts as just one "group" of electrons when figuring out the shape. They all take up about the same amount of space.
Images for kids
-
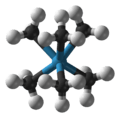
Xenon hexafluoride, which has a distorted octahedral geometry.
See also
 In Spanish: TRePEV para niños
In Spanish: TRePEV para niños
 | Jessica Watkins |
 | Robert Henry Lawrence Jr. |
 | Mae Jemison |
 | Sian Proctor |
 | Guion Bluford |