Brain–computer interface facts for kids
A brain–computer interface (BCI) is a direct way for your brain to talk to an outside device, like a computer or a robotic arm. Think of it as a special connection that lets your thoughts control technology. BCIs are often used to help people, improve their abilities, or fix problems with how their brain controls their body or senses.
Scientists started researching BCIs in the 1970s. The term "brain–computer interface" first appeared in a science paper in 1973. Your brain is amazing and can adapt. This means that signals from special devices put into the brain can eventually be used by the brain almost like natural signals. After many years of testing on animals, the first BCI devices were put into humans in the mid-1990s.
Contents
Animal BCI Research
Many labs have successfully recorded signals from the brains of monkeys and rats. They used these signals to let the animals control BCIs and make movements. Monkeys have learned to move computer cursors on a screen. They have also commanded robotic arms to do simple tasks. They did this just by thinking about the task and seeing what happened, without moving their own bodies.
In May 2008, pictures showed a monkey at the University of Pittsburgh Medical Center controlling a robotic arm just by thinking. These pictures were shared in many famous science magazines. Sheep have also been used to test BCI technology.
In 2020, a company called Neuralink, started by Elon Musk, successfully put a BCI device into a pig. This was shown in a popular online video. In 2021, Elon Musk announced that a monkey was able to play video games using Neuralink's device.
Types of BCIs
BCIs can be put into different groups based on how they connect to the brain.
Invasive BCIs
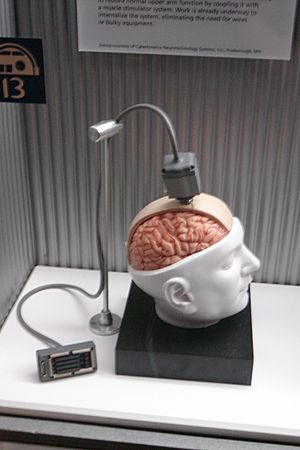
Invasive BCIs need surgery to place tiny electrodes under the scalp or directly into the brain. The main benefit of these is that they get very clear and accurate signals from the brain. However, there are risks with surgery. Sometimes, scar tissue can form after the surgery, which can make the brain signals weaker. Also, the body might not accept the implanted electrodes, which can cause health problems.
Helping Vision
Some invasive BCI research aims to fix damaged sight. It also tries to give new abilities to people who cannot move. Invasive BCIs are placed directly into the brain's grey matter during surgery. Because they are deep inside the brain, they get the best quality signals. But, as mentioned, scar tissue can build up. This can make the signal weaker or even stop it completely. This happens as the body reacts to the device being a foreign object.
One of the first scientists to create a working brain interface to restore sight was William Dobelle. In 1978, Dobelle's first test device was put into "Jerry," a man who became blind as an adult. This BCI had 68 electrodes. It was placed on Jerry's visual cortex, which is the part of the brain that processes sight. The device helped him see "phosphenes," which are sensations of seeing light. The system used cameras on glasses to send signals to the implant.
At first, Jerry could see shades of grey in a small area, and the images were slow. He also had to be connected to a large computer. But as electronics got smaller and computers got faster, his artificial eye became more portable. It eventually allowed him to do simple tasks without help.
In 2002, Jens Naumann, who also became blind as an adult, was one of the first patients to pay for Dobelle's next-generation implant. This was one of the earliest times BCIs were used for commercial purposes. Right after his implant, Jens could use his partly restored vision to slowly drive a car in the research center's parking lot. Sadly, Dobelle passed away in 2004 before he could write down all his discoveries. Later, when Mr. Naumann and other patients started having problems with their vision, there was no help, and they eventually lost their "sight" again.
Helping Movement
BCIs also aim to help people who cannot move their bodies. They can help restore movement or provide devices like computer interfaces or robot arms.
Researchers at Emory University in Atlanta were the first to put a brain implant into a human that produced signals clear enough to help with movement. Their patient, Johnny Ray, had 'locked-in syndrome' after a stroke in 1997. Ray's implant was put in in 1998. He lived long enough to start using the implant and learned to control a computer cursor. He passed away in 2002.
In 2005, Matt Nagle, who was paralyzed from the neck down, became the first person to control an artificial hand using a BCI. This was part of a nine-month human trial of the Cyberkinetics's BrainGate chip. The BrainGate implant was placed in Nagle's brain in the area that controls arm movement. This 96-electrode implant allowed Nagle to control a robotic arm just by thinking about moving his hand. He could also control a computer cursor, lights, and a TV.
More recently, research teams have shown even more success. They have used direct connections to brain cells to control robotic prosthetic limbs. These limbs can move in many different ways.
Helping Communication
In May 2021, a team at Stanford University showed a successful test. A person who was paralyzed could type English sentences at about 86 characters per minute. This is about 18 words per minute. The person imagined moving their hand to write letters. The system then recognized these "imagined" movements from electrical signals in the brain.
Another report in July 2021 shared that a paralyzed patient could communicate 15 words per minute. This was done using a brain implant that looked at brain signals that used to control the voice. In 2023, two studies used BCIs to decode speech at even faster rates: 62 words per minute and 78 words per minute.
Partially Invasive BCIs
Partially invasive BCI devices are put inside the skull. But they rest on the surface of the brain, not deep inside the grey matter. They give clearer signals than non-invasive BCIs. This is because the skull bone can block and change signals. They also have a lower risk of causing scar tissue in the brain compared to fully invasive BCIs.
Non-invasive BCIs
Scientists have also done tests on humans using non-invasive technologies. These devices do not need surgery. Most BCI research uses non-invasive EEG-based BCIs. EEG stands for electroencephalography, which measures brain waves from outside the head.
EEG-based devices are easy to wear and do not need surgery. However, they do not get very detailed signals. This is because the skull can weaken and blur the brain's electrical signals.
Future Directions
BCI research is always moving forward. Here are some exciting areas for the future:
Helping People with Consciousness Disorders
Some people have a disorder of consciousness (DOC). This includes people in a coma or those in a vegetative state (VS) or minimally conscious state (MCS). New BCI research aims to help these people in different ways. A main goal is to find out if these patients can do basic thinking tasks. If they can, it would change their diagnosis. This means some people thought to have DOC might actually be able to understand information. They could even make important life choices, like where to live or if they want therapy.
Also, these patients could get BCI tools to help them communicate basic needs. They could adjust their bed or the room temperature. This would give them more power to make big life decisions and talk to others.
Helping with Movement Recovery
People can lose some ability to move due to many reasons, like a stroke or an injury. Recent research shows that BCI systems can help people recover movement. They can also help with rehabilitation for patients who have had a stroke.
Mapping the Brain
Each year, about 400,000 people have their brains mapped during surgery. This is often needed for people with brain tumors or epilepsy that medicine cannot control. During this procedure, electrodes are placed on the brain. This helps doctors find the exact locations of brain parts and areas that control functions. Patients might be awake during this surgery. They might be asked to do tasks, like moving fingers or repeating words. This helps surgeons remove only the bad tissue. It also helps them avoid damaging important areas, like those for movement or language. Removing too much brain tissue can cause lasting damage. Removing too little can leave the problem untreated. So, there is a big need to make brain mapping methods even better.
The BCI Award
The Annual BCI Research Award is given to recognize amazing and new research in the field of Brain-Computer Interfaces. Each year, a well-known research lab judges the projects that are submitted. The judges are world-leading BCI experts chosen by the awarding lab. They pick twelve nominees. Then, they choose a first, second, and third-place winner. These winners receive awards of $3,000, $2,000, and $1,000, respectively.
Images for kids
See also
 In Spanish: Interfaz cerebro-computadora para niños
In Spanish: Interfaz cerebro-computadora para niños
 | Delilah Pierce |
 | Gordon Parks |
 | Augusta Savage |
 | Charles Ethan Porter |