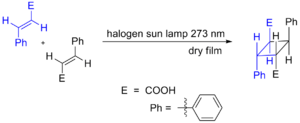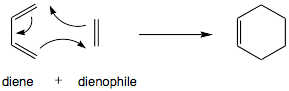Cycloaddition facts for kids
A cycloaddition is a special kind of chemical reaction. It happens when two or more molecules join together to form a new ring-shaped molecule. Think of it like building a new ring out of smaller pieces!
These reactions are named by how many atoms from each starting molecule join the new ring. For example, a Diels–Alder reaction is called a [4 + 2]cycloaddition because it combines a molecule with 4 atoms that form part of the new ring with another molecule that has 2 atoms joining the ring.
Contents
How Cycloadditions Happen
Cycloadditions can be started in a couple of ways:
Using Heat
Sometimes, just adding heat can make these reactions happen. When molecules are heated, their double bonds can rearrange to form a ring. These reactions usually involve a specific number of electrons (called pi electrons) that are involved in forming the new bonds.
Using Light
Light can also make cycloadditions happen! When light hits certain molecules, it can give energy to their electrons. This extra energy allows the molecules to join together and form a ring. An example of this is how cinnamic acid molecules can join together when exposed to light, as shown here:
Types of Cycloadditions
There are different types of cycloaddition reactions, named after the scientists who discovered or studied them.
Diels-Alder Reactions
The Diels–Alder reaction is a very famous type of cycloaddition. It's a [4+2]cycloaddition, meaning one molecule contributes 4 atoms and another contributes 2 atoms to the new ring.
Huisgen Cycloadditions
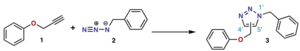
The Huisgen cycloaddition is another important type. It's a [2+3]cycloaddition.
Nitrone-Olefin Cycloaddition
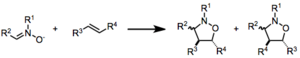
The Nitrone-olefin cycloaddition is a [3+2]cycloaddition.
Formal Cycloadditions
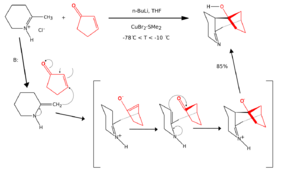
Sometimes, reactions look like cycloadditions but don't follow the exact rules of a "true" cycloaddition. These are called formal cycloadditions. They might involve different steps or intermediates, but the end result is still a new ring-shaped molecule.
Here's an example of a formal [3+3]cycloaddition:
Images for kids
See also
 In Spanish: Cicloadición para niños
In Spanish: Cicloadición para niños