Electrocyclic reaction facts for kids
An electrocyclic reaction is a special type of chemical reaction where a molecule changes its shape by either closing a ring or opening a ring. Imagine a flexible chain of atoms that can link up to form a circle, or a circle that can break open into a chain. That's what happens in these reactions!
These reactions have some cool features:
- They can be started by either light (called photoinduced) or heat (called thermal).
- The way the reaction happens depends on the number of pi electrons in the molecule.
- They can either close a ring (this is called electrocyclization) or open one.
- The final shape of the molecule (its stereospecificity) is very specific. It depends on whether parts of the molecule rotate in the same direction (conrotatory) or in opposite directions (disrotatory). This is predicted by the Woodward–Hoffmann rules.
Sometimes, when a reaction is conrotatory, the parts can still rotate in two different ways. This can lead to a mix of two products that are mirror images of each other. But in some special cases, called torquoselective reactions, the molecule prefers to rotate in only one direction. This means it mostly makes just one of the mirror-image products.
Scientists love studying electrocyclic reactions. They help confirm important ideas in theoretical chemistry about how atoms and their electrons behave.
A famous example of an electrocyclic reaction that closes a ring is the Nazarov cyclization reaction. It changes certain molecules called divinylketones into cyclopentenones. This reaction was discovered by a scientist named Ivan Nikolaevich Nazarov.
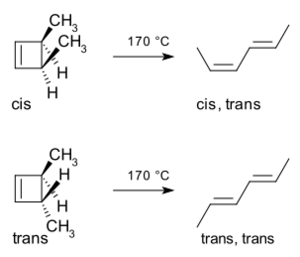
Let's look at an example: the ring-opening reaction of a molecule called 3,4-dimethylcyclobutene. When you heat the cis version of this molecule, it only produces cis,trans-2,4-hexadiene. But if you heat the trans version, it gives you the trans,trans diene.
This happens because the bond that breaks open does so in a very specific way. It makes sure the new parts of the molecule line up correctly. For 3,4-dimethylcyclobutene, the ring opens in a conrotatory way. This means the ends of the breaking bond rotate in the same direction.
The way a molecule rotates (conrotatory or disrotatory) depends on whether the reaction is started by heat or light, and how many electrons are involved. Here's a simple table:
| Type of Molecule | Started by Heat | Started by Light |
|---|---|---|
| "4n" electrons (like 4, 8, 12...) | Conrotatory | Disrotatory |
| "4n + 2" electrons (like 2, 6, 10...) | Disrotatory | Conrotatory |
The final shape of the product molecule is very specific, depending on whether the reaction is conrotatory or disrotatory.
Contents
Woodward-Hoffmann Rules
The Woodward–Hoffmann rules are a set of guidelines that help scientists understand why electrocyclic reactions happen the way they do. They explain how the electron clouds (called orbitals) in a molecule line up and change during the reaction.
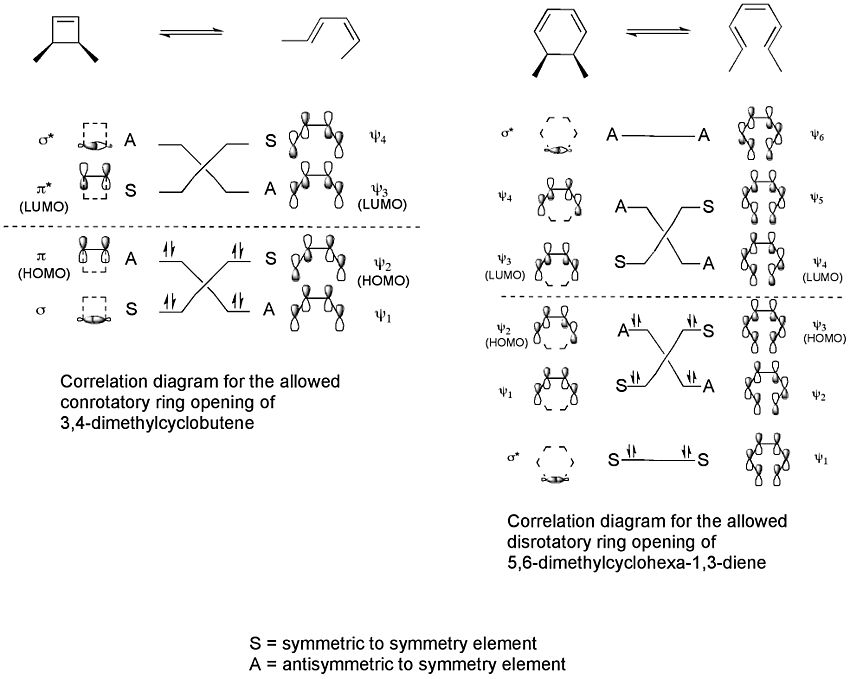
Scientists use something called "correlation diagrams" to show how the electron orbitals of the starting molecule connect to the orbitals of the product molecule. These diagrams help predict if a reaction is "allowed" or "forbidden."
These diagrams show that for 3,4-dimethylcyclobutene, only a conrotatory ring opening is "allowed." This means it's the only way the reaction can happen easily. For another molecule, 5,6-dimethylcyclohexa-1,3-diene, only a disrotatory ring opening is "allowed." This is because these specific rotations allow the electron clouds to overlap perfectly during the reaction. It also ensures the product molecule ends up in a stable state.
Frontier Molecular Orbital Theory
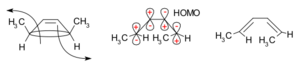
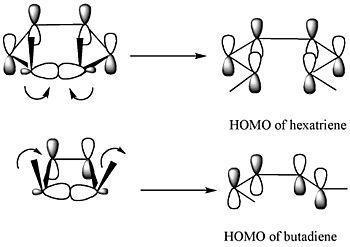
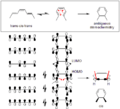
The Frontier Molecular Orbital Theory is another way to predict how these reactions will happen. It focuses on the highest energy electrons in the molecule. It predicts that when a ring opens, the new electron clouds will have the same shape as the highest energy electron cloud of the product molecule.
The diagram above shows two examples. For the molecule 5,6-dimethylcyclohexa-1,3-diene (top part of the diagram), only a disrotatory movement (where the electron clouds rotate in opposite directions) will make the new electron clouds match the product's highest energy electron cloud. For 3,4-dimethylcyclobutene (bottom part of the diagram), only a conrotatory movement (where the electron clouds rotate in the same direction) will work.
Electrocyclic Reactions in Nature
Electrocyclic reactions are not just something scientists do in labs; they happen all the time in nature!
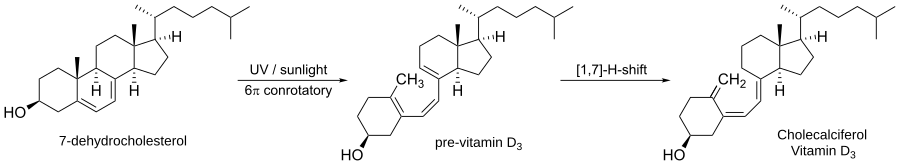
One of the most common examples is how our bodies make vitamin D3.
The first step in making vitamin D3 involves sunlight hitting a molecule in our skin called 7-dehydrocholesterol. This light causes the molecule's ring to open in a conrotatory way. This creates a new molecule called pre-vitamin D3. After that, another step changes pre-vitamin D3 into the vitamin D3 our bodies need.
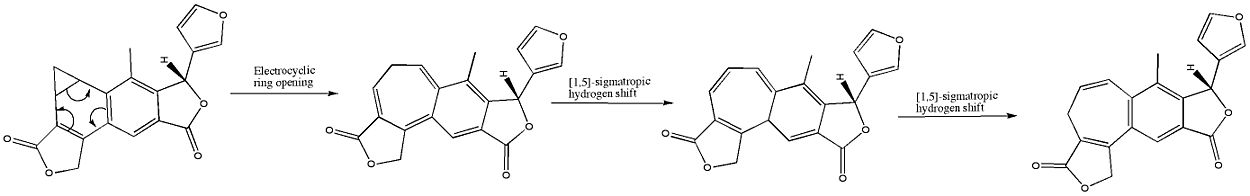
Another example is found in the creation of certain natural compounds like aranotin. In this process, a molecule undergoes a disrotatory ring opening reaction to form a new ring system.
Scientists have also observed these reactions in other natural molecules. For instance, a molecule called benzonorcaradiene diterpenoid (A) can change into isosalvipuberlin (B) when heated. This change involves a disrotatory electrocyclic reaction.
More Examples
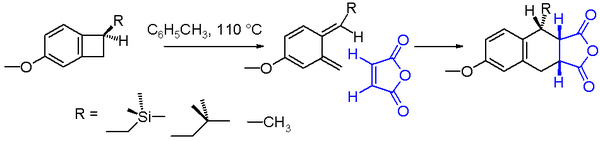
An interesting electrocyclic reaction is the ring-opening of benzocyclobutane when heated. This reaction creates a very unstable molecule called ortho-quinodimethane. Scientists can "trap" this unstable molecule by making it react with another molecule, like maleic anhydride, to form a more stable product.
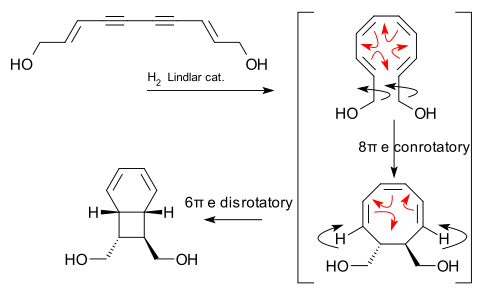
Electrocyclic reactions are also important in making complex natural products in the lab. For example, they play a key role in the creation of certain endiandric acids, which are found in nature.
Images for kids
See also
 In Spanish: Reacción electrocíclica para niños
In Spanish: Reacción electrocíclica para niños