Enthalpy of vaporization facts for kids
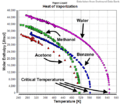
The Enthalpy of vaporization, also known as the latent heat of vaporization, is the amount of energy needed to change a liquid into a gas. This change happens at a specific pressure. We usually measure this energy when a liquid reaches its normal boiling point. The units for this energy are typically Joules per mole (J/mol).
Contents
What is Enthalpy of Vaporization?
When a liquid turns into a gas, it needs energy. Think about boiling water: you have to heat it up a lot before it turns into steam. The enthalpy of vaporization is exactly that energy. It's the hidden (or "latent") heat that a substance absorbs to make this big change from liquid to gas.
How Does it Work?
Imagine water molecules in a liquid. They are close together but can move around. To become a gas, these molecules need enough energy to break free from each other and fly around independently. The enthalpy of vaporization is the energy that helps them do this. It's like giving them a big push to escape the liquid state.
Why is it Important?
This process is very important in everyday life. A great example is how your body stays cool. When you get hot from warm weather or exercise, your body starts to sweat. Sweat is a liquid. As this liquid sweat evaporates from your skin, it takes heat away from your body. This makes you feel cooler.
The faster sweat evaporates, the cooler you feel. If the humidity (the amount of water vapor in the air) is high, sweat evaporates more slowly. This is why you might feel less comfortable and hotter on a humid day, even if the temperature is the same.
Related pages
Images for kids
See also
 In Spanish: Entalpía de vaporización para niños
In Spanish: Entalpía de vaporización para niños