Evaporation facts for kids
Evaporation is a cool process where a liquid turns into a gas. It happens without any bubbles forming in the liquid. Think of it like water slowly disappearing from a puddle on a sunny day.
When water evaporates, it becomes an invisible gas called water vapor. This water vapor then mixes with the air around us. The opposite of evaporation is called condensation. That's when a gas turns back into a liquid.
Inside any liquid, tiny particles called molecules are always moving. When they get enough energy (like from heat), they move faster and faster. Eventually, some molecules escape the liquid and fly off as a gas.

Contents
Evaporation vs. Boiling
Evaporation and boiling both turn a liquid into a gas, but they are different.
During evaporation, only the molecules right at the surface of the liquid change into a gas. This is why you don't see bubbles forming in the liquid.
Boiling is different because molecules throughout the entire liquid turn into a gas. This is why you see lots of bubbles forming and rising in boiling water.
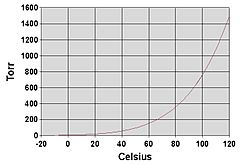
Evaporation can happen at any temperature. For example, water evaporates from a puddle even on a cool day. Boiling, however, only happens at a specific temperature called the "boiling point." For water, this is 100 degrees Celsius (212 degrees Fahrenheit) at sea level.
Evaporation is usually a slow process. Boiling happens very quickly.
How Fast Does Evaporation Happen?
Some liquids evaporate faster than others. Many things can affect how quickly a liquid evaporates:
- Surface Area: The more surface area a liquid has exposed to the air, the faster it evaporates. Think of a wide, shallow dish of water compared to a tall, narrow glass.
- Humidity: If the air is already full of water vapor (high humidity), evaporation slows down. If the air is dry, evaporation speeds up.
- Wind: Wind blows away the water vapor that has just evaporated, making room for more liquid to turn into gas. So, wind makes evaporation faster.
- Temperature: The warmer the liquid, the faster its molecules move and escape into the air. So, higher temperatures mean faster evaporation.
Liquids that have a very high boiling point tend to evaporate more slowly. Liquids with lower boiling points evaporate more quickly.
Evaporation is a super important part of the water cycle on Earth. It helps move water from oceans and lakes into the atmosphere.
Why Evaporation Feels Cool
Evaporation is an endothermic process. This means that when a liquid evaporates, it absorbs heat from its surroundings.
This is why you feel cooler when sweat evaporates from your skin. The evaporating sweat takes heat away from your body.
Everyday Uses of Evaporation
Evaporation is used in many ways, both in nature and by people:
- Drying Clothes: When you hang wet clothes on a line, the water evaporates. This process is sped up by sun, wind, and low humidity. Clothes dryers use hot air to make water evaporate very quickly.
- Cooling Drinks: Traditional clay pots like the Indian Matki or Spanish botijo keep water cool. Water slowly seeps through the porous clay and evaporates from the outside surface, cooling the water inside.
- Evaporative Coolers: These devices cool buildings by blowing dry air over a wet filter. As the water evaporates from the filter, it cools the air, which then cools the room.
- Industrial Uses: Evaporation is used in factories for things like drying lumber, paper, and cloth. It also helps in printing and coating processes, and in getting salts from solutions.
- Science Labs: Scientists use evaporation to dry or concentrate samples for testing.
Fuel Vaporization
In engines, fuel needs to turn into a gas (vaporize) to mix well with air and burn properly.
- In a running engine: Tiny fuel droplets turn into vapor when they mix with hot gases inside the engine's burning chamber.
- Before burning: For an engine to start and run smoothly, the fuel must vaporize into a gas. For example, gasoline needs to mix with air in a specific ratio (about 15 parts air to 1 part gasoline by weight) to burn completely.
Related pages
Images for kids
See also
 In Spanish: Evaporación para niños
In Spanish: Evaporación para niños