Hollow-cathode lamp facts for kids
A hollow-cathode lamp (HCL) is a special kind of light bulb. Scientists use it in physics and chemistry. It helps them study different materials. For example, it's used in machines that check what metals are in a sample.
This lamp works by making light from a specific metal. When tiny parts of a metal, called electrons, get extra energy, they jump to a higher level. This is called excitation. When they fall back down, they release light. HCLs are good at making very clear, specific colors of light. This is useful because each metal gives off its own unique colors.
Contents
How a Hollow-Cathode Lamp Works
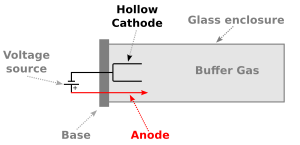
A hollow-cathode lamp is made of a glass tube. Inside this tube are two main parts: an anode and a cathode. The tube is filled with a special gas, like argon or neon. This gas is called an inert gas because it doesn't easily react with other things. This helps keep the light pure.
The anode is a metal part that helps start the process. It's usually made of a metal that doesn't react much. When electricity is turned on, the anode helps to pull electrons from the gas inside the tube. This creates charged gas particles called ions.
The cathode is the most important part. It's shaped like a cup or a hollow cylinder. This cathode is made from the exact metal that scientists want to study. For example, if they want to find lead, the cathode will be made of lead. It's very important to keep the cathode clean and free from dirt. If it gets dirty, the lamp won't work correctly. The glass window of the lamp is also chosen carefully. It lets the right colors of light pass through easily.
Making Light from Metal
When electricity flows through the lamp, the inert gas atoms become charged. These charged gas atoms then crash into the cathode. When they hit the cathode, tiny pieces of the metal from the cathode break off. This process is called sputtering.
These tiny metal pieces then float around inside the lamp. As they move, they get excited by the electricity. This makes their electrons jump to a higher energy level. When these excited electrons fall back to their normal energy level, they release light. This light has very specific colors, which are unique to the metal the cathode is made of. This pure light is then used by scientific instruments.
Keeping the Lamp Working Well
How well a hollow-cathode lamp works depends on how it's used. If too much electricity is put through the lamp, it can make the light very bright. However, this can also cause problems.
Sometimes, too many unexcited metal atoms can build up in the lamp. These atoms can block or absorb the light that the excited atoms are trying to send out. This problem is called self-absorption. It makes the light weaker and less useful for scientific tests.
Using too much electricity can also make the lamp wear out faster. It can even melt the metal on the cathode, which would ruin the lamp completely. So, it's important to use the right amount of electricity to keep the lamp working for a long time.
Images for kids
-
Hollow-cathode lamps from an atomic absorption spectrometer
See also
 In Spanish: Lámpara de cátodo hueco para niños
In Spanish: Lámpara de cátodo hueco para niños