Chemistry facts for kids
Chemistry is a branch of science that studies the things that make up our world. It looks at chemical elements and compounds, and how these things work together. Chemistry helps us understand everything around us, from the air we breathe to the food we eat and even our own bodies!
Contents
History of Chemistry
Before the 1600s, people who studied substances were called alchemists. They tried to do things like turn lead into gold, but they never succeeded. However, alchemists did discover some useful substances, such as sulfuric acid and nitric acid. At that time, only a few elements were known, like mercury, silver, gold, and carbon.
Chemistry became a true science around the 1600s. This is when scientists started to discover the simplest substances that make up everything else. These simple substances are called elements. They learned that gold and lead are different elements, so you can't change one into the other using a chemical reaction. The first new element found after 1600 was phosphorus, a strange white solid that glows.
More and more elements were discovered quickly. Scientists separated air into different parts and found the noble gases. They also processed special rocks from mines to find rare earth metals. The discovery of radioactivity also helped find new elements. Today, chemists have found 118 different elements. Some are very common, like oxygen, which is in the air. Many are very rare and expensive, like platinum. Some elements cannot be found naturally on Earth and can only be made in labs, like rutherfordium.
Since the 1920s, a better understanding of physics has changed how chemists think about chemical reactions. With smaller and faster computers, chemists have created amazing tools to study substances. These tools have even been sent to study chemicals on Mars. Police also use these tools to examine evidence at crime scenes.
Types of Chemistry
There are many different types of chemistry, each focusing on something specific.
- Analytical chemistry helps us find out what chemicals are in things. For example, it can tell us how much arsenic is in food.
- Organic chemistry studies things that contain carbon. An example is making acetylene, which is used in welding.
- Inorganic chemistry looks at substances that do not contain carbon. Making parts for integrated circuits is an example of inorganic chemistry.
A big part of chemistry is polymer chemistry. This area focuses on plastics. Making nylon, which is used in clothes and ropes, is an example. Since plastics are made of carbon, polymer chemistry is actually a part of organic chemistry.
Another important area is biochemistry. This looks at the chemistry of living things. For example, it studies how arsenic can poison people. Biochemistry is also part of organic chemistry. There are many other smaller branches of chemistry too!
Basic Concepts of Chemistry
Building Blocks of Matter
The basic unit of an element is called an atom. An atom is the smallest piece of an element that still keeps its properties. You can't break an atom into smaller parts without changing the element itself.
A chemical compound is a substance made from two or more elements joined together. In a compound, two or more atoms connect to form a molecule. Even the tiniest speck of dust or a single drop of liquid contains millions or billions of these molecules.
Mixtures and Compounds
Mixtures are substances where different chemicals are simply mixed together but not chemically joined. For example, if you mix sand and salt, you can separate them again.
Chemical compounds are different. They are formed when chemicals react and change into new substances. For example, if you heat sodium bicarbonate (baking soda), it changes into water, carbon dioxide, and sodium carbonate. You cannot easily turn these new substances back into baking soda.
Understanding the Mole
A mole is a very, very large number of atoms or molecules. It's about 602,214,150,000,000,000,000,000 atoms! This huge number helps chemists count tiny particles.
The atomic mass of an element tells us how many grams of that element make up one mole of its atoms. For example, the atomic mass of copper is about 63.55. This means that about 63.55 grams of copper metal contains one mole of copper atoms. The atomic mass of chlorine is about 35.45, so 35.45 grams of chlorine has one mole of chlorine atoms.
Moles are also used for chemical compounds. For example, Copper(II) chloride has the chemical formula CuCl2. This means it has one copper atom and two chlorine atoms. To find the mass of one mole of copper(II) chloride, you add the atomic masses: 63.55 (for copper) + 70.90 (for two chlorines) = 134.45 grams. So, 134.45 grams of copper(II) chloride contains one mole of its molecules. Chemists use moles to figure out exactly how much of each chemical they need for a reaction, so nothing is wasted.
Acids and Bases
Acids and bases are common types of chemicals. Acids release special particles called H+ ions when they are in water. Bases release OH− ions when in water.
Acids and bases can react with each other. When they do, the H+ ion from the acid joins with the OH− ion from the base to make water (H2O). This reaction also creates a type of chemical called a salt.
For example, if you mix hydrochloric acid (HCl) and sodium hydroxide (NaOH):
- Hydrochloric acid releases H+ and Cl- ions in water.
- Sodium hydroxide releases Na+ and OH- ions.
- The H+ and OH- ions combine to form water.
- What's left is a solution of sodium chloride (NaCl), which is common table salt. Sodium chloride is a salt.
Usefulness of Chemistry
Chemistry is incredibly useful in our daily lives and is the basis for many other sciences. Most of the objects we use every day are made thanks to chemists. Chemists are always working to discover new and helpful substances. They create new drugs to help us stay healthy and new materials like paints that we use all the time.
Safety in Chemistry
While many chemicals are harmless, some can be dangerous. For example, mercury(II) chloride is very poisonous. Some chemicals like Chromates can increase the risk of cancer. Tin(II) chloride can easily pollute water. Hydrochloric acid can cause serious burns if it touches your skin. Also, some chemicals like hydrogen can explode or catch fire.
To stay safe, chemists work with chemicals in special labs. They use safety equipment like glasses, lab coats, and gloves. They also use special tools to handle chemicals and keep them safely contained. Chemicals used in drugs and in products like bleach are carefully tested to make sure they are safe when used correctly.
Related pages
Images for kids
-
An oil painting of a chemist (Ana Kansky, painted by Henrika Šantel in 1932)
-
A Laboratory at the Institute of Biochemistry, University of Cologne in Germany.
-
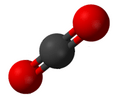
Carbon dioxide (CO2), an example of a chemical compound.
-
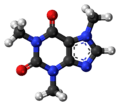
A model of the caffeine molecule (C8H10N4O2).
-
An animation of how sodium (Na) and chlorine (Cl) join to form sodium chloride, or common table salt. This is called ionic bonding.
-
The crystal structure of potassium chloride (KCl), a salt formed by the attraction of K+ and Cl− ions.
-
Democritus' idea that everything is made of tiny atoms was later used by Epicurus (341–270 BCE).
-
A 15th-century drawing of Jābir ibn Hayyān, an early alchemist and chemist.
-
Antoine-Laurent de Lavoisier is often called the "Father of Modern Chemistry".
-
In his periodic table, Dmitri Mendeleev predicted new elements and organized the 60 elements known at the time.
See also
 In Spanish: Química para niños
In Spanish: Química para niños
 | Georgia Louise Harris Brown |
 | Julian Abele |
 | Norma Merrick Sklarek |
 | William Sidney Pittman |