Sodium carbonate facts for kids
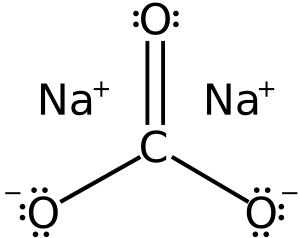
Sodium carbonate is a common chemical compound that you might know as soda ash or washing soda. It's made up of two different parts: sodium and carbonate ions. An ion is like a tiny particle that has an electric charge. The chemical formula for sodium carbonate is Na2CO3. This means it has two sodium atoms (Na) and one carbonate group (CO3).
Sodium carbonate is a type of base. Bases are the opposite of acids. When sodium carbonate mixes with an acid, they react and create carbon dioxide gas. This is the same gas we breathe out!
You can make sodium carbonate in a few ways. One way is by mixing sodium hydroxide with carbon dioxide. Another way is by heating sodium bicarbonate, which is also known as baking soda. For making large amounts, companies use a special method called the Solvay process.
Contents
What is Sodium Carbonate Used For?
Sodium carbonate is a very useful chemical. It's used in many different products and processes.
Making Glass
One of the biggest uses for sodium carbonate is in making glass. It helps to lower the melting point of sand, which is the main ingredient in glass. This makes it easier and cheaper to produce glass for windows, bottles, and other items.
In Pools and Cleaning
Sodium carbonate is also used to help keep swimming pools clean. It helps to adjust the water's pH level, making it safer and more comfortable for swimmers. Because it's a base, it can make the water less acidic. It's also found in some cleaning products, especially those for laundry, because it helps to soften water and remove stains.
Other Uses
- Cooking: Sometimes, it's used in small amounts in cooking, especially in making certain types of noodles or pretzels, to give them a special texture.
- Electrolyte: It can act as an electrolyte. This means it can help electricity move through a solution.
Related Pages
Images for kids
See also
 In Spanish: Carbonato de sodio para niños
In Spanish: Carbonato de sodio para niños
 | Anna J. Cooper |
 | Mary McLeod Bethune |
 | Lillie Mae Bradford |