Sodium hydroxide facts for kids
Quick facts for kids Sodium hydroxide |
|
|---|---|
 |
|
 |
|
| IUPAC name | Sodium hydroxide |
| Other names |
|
| Identifiers | |
| CAS number | |
| PubChem | |
| EC number | 215-185-5 |
| KEGG | D01169 |
| MeSH | |
| ChEBI | CHEBI:32145 |
| RTECS number | WB4900000 |
| SMILES | [OH-].[Na+] |
|
InChI
InChI=1/Na.H2O/h;1H2/q+1;/p-1
|
|
| Gmelin Reference | 68430 |
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | White, opaque crystals |
| Odor | odorless |
| Density | 2.13 g/cm3 |
| Melting point | |
| Boiling point | |
| 418 g/L (0 °C) 1000 g/L (25 °C) 3370 g/L (100 °C) |
|
| Solubility | soluble in glycerol, negligible in ammonia, insoluble in ether, slowly soluble in propylene glycol |
| Solubility in methanol | 238 g/L |
| Solubility in ethanol | 139 g/L |
| Vapor pressure | <2.4 kPa (20 °C) 0.1 kPa (700 °C) |
| Acidity (pKa) | 13.9 (Na+) |
| Basicity (pKb) | 0.0 (OH−) |
| −15.8·10−6 cm3/mol (aq.) | |
| Refractive index (nD) | 1.3576 |
| Structure | |
| Crystal structure | Orthorhombic, oS8 |
| Space group | Cmcm, No. 63 |
| Lattice constant | a = 0.34013 nm, b = 1.1378 nm, c = 0.33984 nm |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
−425.8 kJ/mol |
| Standard molar entropy S |
64.4 J/(mol·K) |
| Specific heat capacity, C | 59.5 J/(mol·K) |
| Hazards | |
| NFPA 704 |
|
| U.S. Permissible exposure limit (PEL) |
TWA 2 mg/m3 |
| Related compounds | |
| Other anions |
|
| Other cations |
|
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Sodium hydroxide, also known as lye or caustic soda, is a common chemical compound. Its scientific formula is NaOH. It looks like a white solid and is made of tiny charged particles called ions: sodium ions (Na+
) and hydroxide ions (OH−
).
Sodium hydroxide is a very strong base and alkali. This means it can be very dangerous and cause serious chemical burns if it touches your skin or eyes. It can break down fats and proteins, even at normal temperatures.
It dissolves very easily in water and can even pull moisture and carbon dioxide from the air. When it mixes with water, it can form different types of hydrates (compounds with water molecules). The kind you often find in stores is a monohydrate, meaning it has one water molecule attached.
In chemistry classes, sodium hydroxide is often used with water and hydrochloric acid to show how the pH scale works, helping students understand acids and bases. This chemical is super useful! Industries use it to make wood pulp and paper, textiles, soaps, and detergents. It's also a common ingredient in drain cleaners. In 2022, about 83 million tons of sodium hydroxide were produced around the world.
Contents
What is Sodium Hydroxide Like?
Physical Characteristics
Pure sodium hydroxide is a clear, solid crystal. It melts at 318 °C (604 °F) and boils at 1388 °C (2530 °F). It dissolves very well in water. It also dissolves in some other liquids like ethanol and methanol, but not in others like ether.
When solid sodium hydroxide dissolves in water, it creates a lot of heat. This is called an exothermic reaction. Because of the heat, it can be dangerous and might splash. The solution is usually clear and has no smell. If it touches your skin, it feels slippery. This is because it reacts with the natural oils on your skin to make a type of soap.
How Thick is it?
Solutions of sodium hydroxide can be quite thick, or viscous. A strong solution (50%) is much thicker than water, almost like olive oil. The warmer the solution, the thinner it becomes. This thickness is important for how it's used and stored.
Sodium Hydroxide and Water
Sodium hydroxide can combine with water molecules to form different types of hydrates. These are like different versions of the compound that include water. The way these hydrates form depends on the temperature and how much sodium hydroxide is dissolved in the water. For example, the most common one found in stores is a monohydrate, meaning it has one water molecule for each sodium hydroxide molecule.
Crystal Shape
When sodium hydroxide forms a solid, its tiny particles arrange themselves in a specific pattern called a crystal structure. Both pure sodium hydroxide and its monohydrate form crystals with a special shape called 'orthorhombic'. In these crystals, each sodium atom is surrounded by oxygen atoms, creating a layered structure.
Chemical Reactions
Reacting with Acids
Sodium hydroxide is a strong base, so it reacts strongly with acids. When it mixes with an acid, they neutralize each other, creating water and a salt. For example, if you mix sodium hydroxide with hydrochloric acid, you get sodium chloride (table salt) and water. This reaction also releases heat. Because sodium hydroxide absorbs water and carbon dioxide from the air, it's not used for very precise measurements in some experiments.
Reacting with Acidic Gases
Sodium hydroxide can also react with certain oxides that are acidic, like sulfur dioxide. This reaction is useful in factories to 'clean' harmful gases from the air, especially those produced when burning coal. It helps stop these gases from polluting our atmosphere.
Reacting with Metals
Sodium hydroxide can slowly react with glass, which is why it can damage glass containers over time. It doesn't usually react with iron at normal temperatures. However, it reacts strongly with some other metals, like aluminium. For example, in 1986, a truck carrying aluminum was accidentally filled with sodium hydroxide. This caused a dangerous reaction, producing hydrogen gas and damaging the truck. This is why it's important to store chemicals in the right containers!
Making Solids Appear
Sodium hydroxide can be used to make certain metal compounds separate out of a liquid solution as a solid. This process is called precipitation. For example, when you add sodium hydroxide to solutions containing copper, iron(II), or iron(III), you'll see different colored solids form: blue for copper, green for iron(II), and yellow/brown for iron(III). This property is also used in water treatment to help filter out tiny particles from water.
Making Soap (Saponification)
Sodium hydroxide is very important for making soap! This process is called saponification. It works by breaking down fats and oils. If you accidentally touch a sodium hydroxide solution, it feels slippery because it's turning the natural oils on your skin into soap. (But remember, you should never touch it with bare hands because it's dangerous!)
How Sodium Hydroxide is Made
Most sodium hydroxide is made in factories using a process called the chloralkali process. This method uses electricity to separate sodium chloride (table salt) into sodium hydroxide, chlorine gas, and hydrogen gas. It's first made as a liquid solution, then water is removed to create solid forms like flakes or pellets.
In 2022, about 83 million tons of sodium hydroxide were produced worldwide. Major producers are in North America and Asia. Historically, it was made by mixing sodium carbonate with calcium hydroxide. Another way to make it is by mixing pure sodium metal with water, which creates a lot of heat and hydrogen gas. This reaction is often shown in science classes to demonstrate how reactive alkali metals are.
What is Sodium Hydroxide Used For?
Sodium hydroxide is a very useful chemical in many industries. It helps make sodium salts and detergents, controls pH levels, and is used in creating many organic chemicals. It's often used as a liquid solution because it's cheaper and easier to handle.
In the Petroleum Industry
In the oil industry, sodium hydroxide is added to drilling mud to make it more alkaline. This helps control the mud's thickness and neutralizes any acidic gases found during drilling. It's also used in salt spray testing to balance pH.
Cleaning Crude Oil
Poor quality crude oil can be cleaned with sodium hydroxide to remove impurities like sulfur. This process is called 'caustic washing'. The sodium hydroxide reacts with weak acids in the oil, turning them into salts that can be removed. However, the waste from this process is toxic, so it's banned in many places.
Everyday Uses
- Making Soaps and Detergents: It's used for making solid bar soaps.
- Drain Cleaners: It helps unblock drains by turning fats and grease into soap that dissolves in water.
- Textiles: Used to make artificial fibers like rayon.
- Paper Production: About a quarter of all industrial sodium hydroxide goes into making paper.
- Aluminium Production: It helps purify bauxite ore to get aluminium.
- Metal Cleaning: Used to degrease metals.
- Dyes and Bleaches: An ingredient in making colors and bleaches.
- Water Treatment: Helps adjust the pH of water supplies, making water less corrosive to pipes.
- Food Preparation: Used to wash fruits and vegetables, process chocolate, and give pretzels their shiny crust. It's known as E524 in food.
Making Paper Pulp
Sodium hydroxide is widely used to turn wood into paper or regenerated fibers. It's a key part of the 'white liquor' solution that separates lignin from cellulose fibers in the kraft process. It also helps in later steps to bleach the brown pulp, requiring a strong alkaline environment.
Breaking Down Organic Materials
Sodium hydroxide can break down organic tissues. This process has been used in controlled industrial settings, for example, to dispose of animal remains. It turns the material into a liquid, leaving only bone fragments.
Dissolving Special Metals
Strong bases like sodium hydroxide can attack certain metals, such as aluminium. When sodium hydroxide reacts with aluminium and water, it releases hydrogen gas. This reaction creates sodium aluminate. This can be useful for etching or cleaning aluminium surfaces.
In the Bayer process, sodium hydroxide helps refine bauxite ore to produce alumina (aluminium oxide). Alumina is then used to make aluminium metal. The sodium hydroxide dissolves the alumina, leaving impurities behind. Other metals like zinc and lead also dissolve in strong sodium hydroxide solutions.
Making Biodiesel
Sodium hydroxide acts as a catalyst (something that speeds up a reaction) in making biodiesel. It helps change methanol and triglycerides into biodiesel. It's often preferred over potassium hydroxide because it's cheaper.
In Skincare Products
Sodium hydroxide is found in some skin care and cosmetic products, like facial cleansers and lotions. It's used in very small amounts to balance the pH level of the product.
Cleaning Agent
Sodium hydroxide is a common industrial cleaning agent, often called "caustic". It's added to hot water to clean equipment and storage tanks. It can dissolve grease, oils, fats, and protein-based dirt. It's also used in oven cleaners and to clean clogged drains at home. When it dissolves in water, it creates a lot of heat, which helps it work faster. These cleaners are very corrosive and must be handled with extreme care.
Hair Relaxers
Sodium hydroxide is used in some hair relaxers to straighten hair. However, because it can cause severe chemical burns, other alkaline chemicals are often used in products available to consumers. Sodium hydroxide relaxers are mostly used by professionals.
Water Purification
Sodium hydroxide is sometimes used in water purification to raise the pH of water supplies. A higher pH makes the water less corrosive to pipes, which reduces the amount of harmful metals like lead and copper that can dissolve into drinking water.
Historical Uses
In the past, sodium hydroxide was used to detect carbon monoxide poisoning. Blood samples from affected patients would turn a red color when a few drops of sodium hydroxide were added. Today, more advanced methods are used.
In Building Materials
Sodium hydroxide is used in some cement mix plasticizers. This helps make cement mixes smoother, prevents sand and cement from separating, reduces the amount of water needed, and makes the final product easier to work with.
Safety First!
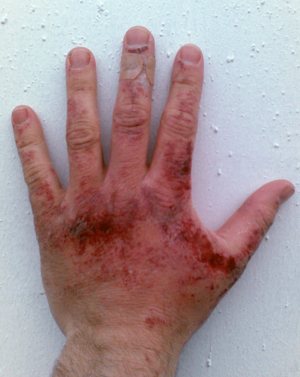
Sodium hydroxide is a very dangerous chemical. Like other strong acids and alkalis, even a few drops can cause severe chemical burns if it touches your skin or eyes. It can break down proteins and fats in living tissues, which can lead to permanent damage, including blindness if it gets into your eyes.
Always wear protective equipment like rubber gloves, safety clothing, and eye protection when handling sodium hydroxide or its solutions. If it spills on your skin, immediately wash the area with large amounts of running water for at least ten to fifteen minutes.
Remember, dissolving sodium hydroxide in water creates a lot of heat, which can cause heat burns or even ignite flammable materials nearby. It also produces heat when it reacts with acids. Sodium hydroxide can also corrode some metals, like aluminium, producing flammable hydrogen gas.
It's important to know that sodium hydroxide can be harmful to fish and other aquatic life if it gets into water in large amounts, as it changes the water's pH. However, it usually gets neutralized quickly and doesn't stay in the environment for long.
How to Store Sodium Hydroxide
Storing sodium hydroxide safely is very important, especially for large amounts. Always follow proper storage guidelines to protect workers and the environment.
For small amounts, it's stored in bottles in laboratories. Larger amounts are kept in special containers for transport or in huge storage tanks at factories. Materials that work well for storing sodium hydroxide include polyethylene, carbon steel, polyvinyl chloride (PVC), stainless steel, and fiberglass reinforced plastic.
Sodium hydroxide must be stored in airtight containers. This is because it absorbs water and carbon dioxide from the air, which can change its strength and properties.
A Look Back: History of Sodium Hydroxide
Sodium hydroxide was first made by soap makers. A recipe for making it appeared in an Arab book from the late 13th century. This recipe involved passing water through a mixture of 'alkali' (ash from certain plants rich in sodium) and quicklime (calcium oxide). This process created a solution of sodium hydroxide.
European soap makers also used this method. In 1791, a French chemist named Nicolas Leblanc found a way to mass-produce sodium carbonate, which then replaced the natural plant ash. However, by the 20th century, the chloralkali process (using electricity to separate salt) became the main way to produce sodium hydroxide, as it was more efficient.
See Also
 In Spanish: Hidróxido de sodio para niños
In Spanish: Hidróxido de sodio para niños
- Acids and Bases
- List of cleaning agents
 | Selma Burke |
 | Pauline Powell Burns |
 | Frederick J. Brown |
 | Robert Blackburn |





