Saponification facts for kids
Saponification is a cool chemical process. It's how we make soap! Imagine taking a type of chemical called an ester and breaking it apart. When you do this using a strong water solution of an alkali (like sodium hydroxide, also known as lye), you get two new things: a carboxylate salt (which is soap if it's a long chain!) and an alcohol.
Think of it like this: C
2H
5O
2CCH
3 + NaOH → C
2H
5OH + NaO
2CCH
3 This shows that ethyl acetate plus sodium hydroxide gives you ethanol and sodium acetate. It's a key way to make many useful products.
Contents
Making Soap from Fats
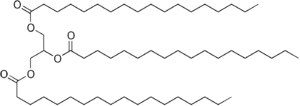
Have you ever wondered how soap is made? It often starts with Vegetable oils or animal fats. These fats are special types of esters called triglycerides. They are usually a mix of different fatty acids.
In the traditional soap-making process, we mix these fats with lye (a strong alkali). The lye breaks the bonds in the fats. This releases fatty acid salts, which are the soaps! It also creates glycerol.
Here's a simplified example using stearin, a common fat: C
3H
5(O
2C(CH
2)
16CH
3)
3 + 3 NaOH → C
3H
5(OH)
3 + 3 NaO
2C(CH
2)
16CH
3 This reaction makes glycerol (C
3H
5(OH)
3) and sodium stearate (a type of soap). Making glycerol is a big part of this process in factories.
Some people who make soap like to leave the glycerol in the soap. This can make the soap feel nicer on your skin. Other times, the soap is separated from the glycerol by adding sodium chloride (table salt). This makes the soap "salt out" or precipitate (form a solid).
What is Saponification Value?
The saponification value tells us how much alkali (like lye) is needed to turn a certain amount of fat into soap. Soap makers use this value to figure out how much lye to add to their oils. Sometimes, they use a little less lye than the exact amount. This makes sure all the lye reacts and there's no extra left in the soap.
How Does Saponification Work?
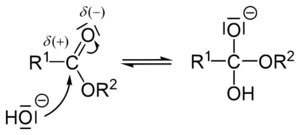
Let's look at the basic steps of how this chemical reaction happens. First, a hydroxide ion (from the alkali) attacks a part of the ester called the carbonyl group. This creates a new, temporary molecule.
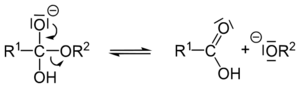
Next, a part of the molecule called an alkoxide breaks off. This leaves behind a carboxylic acid.
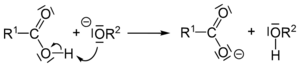
Finally, the alkoxide ion is a very strong base. It takes a proton (a tiny part of an atom) from the carboxylic acid. This turns the carboxylic acid into a carboxylate ion (which is the soap!) and the alkoxide into an alcohol.
In a classic lab experiment, a fat called trimyristin is taken from nutmeg. Then, it's turned into soap (sodium myristate) using NaOH in water. If you then add hydrochloric acid to this soap, you get myristic acid.
Making Soap from Fatty Acids
Another way to make soap is by reacting fatty acids directly with a base. This process is called neutralization. It's like the base "cancels out" the acid. This method is often used to make special industrial soaps. These include soaps made with magnesium, transition metals, or aluminium.
This way of making soap is great for creating soaps from just one type of fatty acid. This means the soaps will have very specific properties, which is important for many engineering uses.
Cool Uses of Saponification
Hard and Soft Soaps
The type of alkali used in soap making changes how the soap behaves.
- If you use Sodium hydroxide (NaOH), you get "hard" soaps. These soaps work well even in water that has minerals like magnesium, chlorine, and calcium.
- If you use KOH, you get "soft" soaps.
The type of fat or oil used also affects the soap's melting point. Older hard soaps were often made from animal fats and KOH from wood ash. These were usually solid. But most modern soaps are made from vegetable oils. These oils have polyunsaturated triglycerides. The soaps made from them have weaker forces between their molecules, so they melt at lower temperatures.
Lithium Soaps
Special soaps like Lithium 12-hydroxystearate are very important in lubricating greases. These lithium-based soaps help make the grease thick. "Complex soaps" are also common. These are made from more than one type of acid salt, like azelaic or acetic acid.
Fire Extinguishers
Did you know saponification can help put out fires? Fires involving cooking fats and oils (like in a kitchen) burn very hot. A regular fire extinguisher might not work.
That's where "wet chemical" fire extinguishers come in. They are designed to put out these types of fires using saponification. The special chemical in the extinguisher quickly turns the burning oil or fat into a non-burning soap. This stops the fire!
Saponification in Art
Saponification can even happen in old oil paintings! Over time, it can cause damage. Oil paints are made of tiny color particles (pigments) mixed in an oil medium. Some pigments contain heavy metals like lead or zinc. If these metals react with fatty acids in the oil, they can form "metal soaps." These soaps can then move to the surface of the painting.
People have known about saponification in oil paintings since at least 1912. It's thought to be quite common in paintings from the 15th to the 20th centuries. It's found in art from different places and on different surfaces, like canvas, paper, wood, and copper. Scientists can even find signs of saponification deep inside a painting before any damage is visible on the surface.
When saponification happens, it can make the painting's surface bumpy or lumpy. These lumps can make the painting look different when light hits it. For example, in John Singer Sargent's famous Portrait of Madame X, lumps appeared only on the black parts of the dress. This might be because the artist used more oil medium in those areas. The process can also create chalky white spots on the surface, or make some paint layers more see-through over time.
Saponification doesn't happen in all oil paintings, and scientists are still learning about it. For now, fixing the damage usually involves carefully restoring the painting.
See also
- Soap
- Saponification value
- Ester hydrolysis
 | Jewel Prestage |
 | Ella Baker |
 | Fannie Lou Hamer |