Sulfur facts for kids
Sulfur (also spelled sulphur) is a chemical element. Its symbol is S, and its atomic number is 16. This means each sulfur atom has 16 protons. Sulfur is a common element found all over the Earth.
Contents
What Is Sulfur Like?
Sulfur is a bright yellow solid. It is a nonmetal, which means it does not conduct electricity or heat well. It is also brittle, so it breaks easily. Sulfur often forms crystals.
When sulfur burns, it gives off poisonous fumes of sulfur dioxide. These fumes can be harmful to breathe. Sulfur itself has only a very faint smell. The strong "sulfur smell" people often talk about actually comes from other chemicals, like hydrogen sulfide. These chemicals are made when things rot without air.
If you melt sulfur and cool it very quickly, it turns into a rubbery material called "plastic sulfur." Over time, this rubbery form slowly changes back into its usual yellow, brittle state. Sulfur does not dissolve in water.
Sulfur Compounds
Sulfur can combine with other elements to form many different chemical compounds. These compounds are found everywhere, from nature to everyday products.
Sulfur Gases
Some sulfur compounds are gases.
- Sulfur dioxide is a colorless, heavy gas. It is toxic and can be used to keep dried foods fresh.
- Sulfur trioxide can be found in different forms, sometimes even as a liquid. It is irritating and toxic.
Sulfur Acids
When sulfur oxides mix with water, they can form acids.
- Sulfuric acid is a very strong acid. It is one of the most used chemicals in the world, after water. It can even turn paper black!
- Hydrogen sulfide is a gas that smells like rotten eggs. When it dissolves in water, it becomes acidic.
Sulfur Salts
Sulfur also forms many types of salts.
- Sulfides are salts of hydrogen sulfide. An example is iron sulfide, which is found naturally as a mineral called pyrite.
- Sulfites are salts of sulfurous acid. Sodium sulfite is used to help preserve dried foods.
- Sulfates are salts of sulfuric acid. Many common substances are sulfates. For example, barium sulfate is used in medical X-rays of the digestive system. Copper(II) sulfate is used to kill algae.
Where Is Sulfur Found?
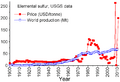
Sulfur can be found naturally near volcanoes. Many minerals in the Earth's crust contain sulfur. Coal also contains sulfur, which is released when coal burns. To get sulfur from the ground, it is often melted and then pushed up through pipes using compressed air.
How Is Sulfur Used?
Sulfur has many important uses.
- It is a key ingredient in gunpowder.
- It is used in some medicines.
- Matches contain sulfur, and the smell you notice when a match burns is from sulfur dioxide.
- Sulfur is vital for all living things. Many proteins in our bodies contain sulfur.
- It is also used as a pesticide on organic farms to protect crops from pests.
History of Sulfur
The ancient name for sulfur was brimstone. People in ancient Greece used sulfur for fumigation (creating fumes to clean or disinfect) and in medicine. In 1777, a scientist named Antoine Lavoisier showed the scientific world that sulfur was truly a chemical element.
Is Sulfur Safe?
Pure sulfur itself is not very toxic. However, the chemical compounds that form when sulfur burns can be very dangerous. For example, sulfuric acid is a strong chemical that can cause serious burns.
Related Pages
Images for kids
-
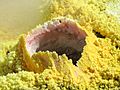
Sulfur is found in fumaroles (gas vents) like this one in Vulcano, Italy.
-
Sulfur recovered from oil and gas in Alberta, Canada, stored for shipping in North Vancouver.
-
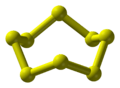
Two parallel sulfur chains grown inside a single-wall carbon nanotube.
-
Allicin, the active ingredient found in garlic.
See also
 In Spanish: Azufre para niños
In Spanish: Azufre para niños
 | William L. Dawson |
 | W. E. B. Du Bois |
 | Harry Belafonte |