Atomic number facts for kids
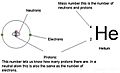
The atomic number (symbol: Z) is a special number for every atom. It tells you how many tiny particles called protons are inside the atom's center, called the nucleus. This number is super important because it identifies what element the atom is. For example, all carbon atoms have an atomic number of 6, meaning they all have 6 protons.
In an atom that has no electric charge (a neutral atom), the atomic number also equals the number of electrons orbiting the nucleus. The elements on the periodic table are arranged in order of their atomic number, starting from 1 (hydrogen) and going up.
Contents
What is Atomic Number?
The atomic number is like an atom's unique ID card. It's the number of protons in its nucleus. If you change the number of protons, you change the element itself! For instance, an atom with 6 protons is always carbon. If it suddenly had 7 protons, it would become nitrogen.
Atomic Number vs. Other Numbers
It's easy to mix up the atomic number with other terms. Here's how they are different:
- Atomic mass (symbol: ma): This is the actual weight of a single atom. It's usually measured in very tiny units called unified atomic mass units.
- Mass number (symbol: A): This is the total count of protons and neutrons in an atom's nucleus. It tells you how heavy the nucleus is.
- Relative atomic mass (also called atomic weight; symbol: Ar): This is an average mass for an element. It compares the average weight of an element's atoms to 1/12th the mass of a carbon-12 atom.
Protons, Electrons, and Neutrons
The atomic number is all about protons. But what about electrons and neutrons?
- Protons: As we learned, changing the number of protons changes the element.
- Electrons: Adding or removing electrons changes an atom's electric charge. If an atom gains electrons, it becomes negatively charged. If it loses electrons, it becomes positively charged. These charged atoms are called ions. Metals often lose electrons, becoming positive ions. Non-metals tend to gain electrons, becoming negative ions. Electrons are key to how atoms connect and form compounds.
- Neutrons: Adding or removing neutrons changes an atom's isotope. An isotope is a different version of the same element. For example, carbon-12 is the most common and stable form of carbon, with 6 protons and 6 neutrons. If you add two more neutrons, it becomes carbon-14, which is a less stable isotope of carbon. The number of neutrons in an atom can be found by subtracting the atomic number (protons) from the mass number (protons + neutrons).
Related pages
Images for kids
-
Russian chemist Dmitri Mendeleev, creator of the periodic table.
-
Niels Bohr, creator of the Bohr model.
-
Henry Moseley in his lab.
See also
 In Spanish: Número atómico para niños
In Spanish: Número atómico para niños
 | Jackie Robinson |
 | Jack Johnson |
 | Althea Gibson |
 | Arthur Ashe |
 | Muhammad Ali |