Atom facts for kids
Quick facts for kids Helium atom |
|
|---|---|
|
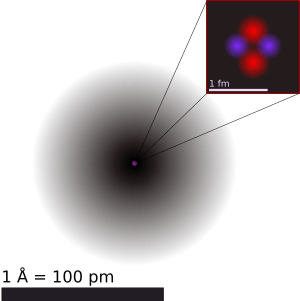
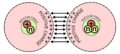
An illustration of the helium atom, depicting the nucleus (pink) and the electron cloud distribution (black). The nucleus (upper right) in helium-4 is in reality spherically symmetric and closely resembles the electron cloud, although for more complicated nuclei this is not always the case. The black bar is one angstrom (10−10 m or 100 pm).
|
|
| Classification | |
| Smallest recognized division of a chemical element | |
| Properties | |
| Mass range | 1.67×10−27 to 4.52×10−25 kg |
| Electric charge | zero (neutral), or ion charge |
| Diameter range | 62 pm (He) to 520 pm (Cs) (data page) |
| Components | Electrons and a compact nucleus of protons and neutrons |
Atoms are super tiny building blocks that make up everything around you! Imagine the smallest piece of anything – that's an atom. There are many different kinds of atoms, and each kind is called a chemical element. You can find these elements organized on the periodic table. Some examples are hydrogen and gold.
Atoms are incredibly small. Their size depends on the element, but they are usually between 0.1 and 0.5 nanometers wide. To give you an idea, one nanometer is about 100,000 times smaller than a human hair! Because they are so tiny, you can't see atoms without special tools. Scientists learn about them by doing experiments to see how they work and interact.
Atoms can also join together to create molecules. For example, two hydrogen atoms and one oxygen atom combine to make a water molecule. When atoms connect like this, it's called a chemical reaction.
Atoms are made of even smaller pieces called protons, neutrons, and electrons. Protons and neutrons are heavier and stay in the center of the atom. This center part is called the nucleus. The nucleus is surrounded by a cloud of very light electrons. These electrons are pulled towards the protons in the nucleus because they have opposite electric charges. This pull is called the electromagnetic force.
The number of protons in an atom tells you what chemical element it is. This number is also known as its atomic number. For instance, hydrogen always has one proton, and sulfur always has 16 protons. Protons and neutrons have similar weight, while electrons are much lighter. So, the total number of protons and neutrons in an atom gives us its atomic mass.
Atoms move differently depending on whether they are in a solid, liquid, or gas. In a gas, atoms move around very fast because they have lots of space. In a liquid, they can slide past each other. But in solid materials, atoms are packed tightly together. They can only vibrate in place because there's no room for them to move around freely.
Contents
Discovering the Atom's Secrets
Early Ideas About Atoms
The word "atom" comes from the Greek word "atomos," which means "indivisible" or "cannot be cut." The idea of atoms first came from the Greek philosopher Democritus, way back around 400 BC. For a long time, the idea of atoms was mostly a philosophical thought, without much scientific study. This changed when chemistry started to develop in the 1650s.
In 1777, a French chemist named Antoine Lavoisier first defined what an element was. He said an element is a basic substance that cannot be broken down into simpler substances using chemistry. Anything that could be broken down was called a compound.
In 1803, English philosopher John Dalton suggested that elements were made of tiny, solid balls called atoms. Dalton believed that all atoms of the same element had the same mass. He also said that compounds form when atoms of different elements combine in specific ways.
Proving Atoms Exist
In 1827, British scientist Robert Brown observed pollen grains in water under his microscope. He noticed the pollen grains were constantly jiggling. Brown used Dalton's atomic theory to explain this movement, which was named brownian motion. Later, in 1905, Albert Einstein used math to prove that these random movements were indeed caused by the reactions of atoms. This finally proved that atoms truly exist!
In 1869, Russian scientist Dmitri Mendeleev created the first version of the periodic table. This table organizes elements by their atomic number (the number of protons they have). Elements in the same column, or group, usually have similar properties. For example, helium, neon, argon, krypton, and xenon are all in the same column. They are all colorless, odorless gases that don't easily combine with other atoms. They are known as the noble gases.
Uncovering Atom's Inner Parts
J. J. Thomson was the first person to discover electrons in 1897. He found that electrons have a negative charge, unlike protons (positive) and neutrons (no charge). Thomson then proposed the plum pudding model for the atom. He thought an atom was like plum pudding, with electrons (the "plums") stuck in a positive "pudding" mass.
In 1909, Ernest Rutherford did an experiment that showed most of an atom is empty space. He shot tiny particles at a thin gold foil. Many particles went straight through, proving that atoms are mostly empty. He found that the atom's mass is concentrated in a very small center called the atomic nucleus. Electrons are so tiny they make up only about 1% of an atom's mass.
In 1913, Niels Bohr introduced the Bohr model. This model showed that electrons travel around the nucleus in fixed circular paths, like planets orbiting the sun. This was a more accurate idea than Rutherford's model, but scientists have improved upon it since then.
Discovering Isotopes and Neutrons
In 1925, chemist Frederick Soddy found that some elements could have different kinds of atoms. For example, all atoms with two protons are helium atoms. But some helium atoms have two neutrons, while others might have only one. Soddy called these different versions of an element isotopes. We name isotopes by adding the total number of protons and neutrons to the element's name. So, helium with two protons and one neutron is helium-3. Carbon with six protons and six neutrons is carbon-12.
When Soddy developed his idea, he wasn't sure if neutrons actually existed. To prove they were real, physicist James Chadwick and his team created the mass spectrometer. This machine measures the mass of individual atoms. By using it, Chadwick proved that neutrons must exist to account for the atom's total weight.
Splitting and Joining Atoms
In 1937, German chemist Otto Hahn was the first to create nuclear fission in a lab. He was trying to make a new isotope by shooting neutrons at a uranium atom. But instead, the uranium atom split into a smaller barium atom. Hahn had "broken" the uranium atom! This was the first recorded nuclear fission reaction. This discovery eventually led to the creation of the atomic bomb.
Later in the 20th century, physicists used particle accelerators to learn even more about atoms. They found that protons and neutrons are actually made of even smaller particles called quarks.
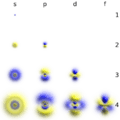
The most accurate model of the atom so far comes from the Schrödinger equation. This model shows that electrons exist in a "cloud" around the nucleus, called the electron cloud. In this cloud, you can't know exactly where an electron is, but the Schrödinger equation helps find where an electron is most likely to be. This area is called the electron's orbital.
What Are Atoms Made Of?
The Main Parts
A complex atom has three main particles: the proton, the neutron, and the electron. Protons have a positive electric charge, and electrons have a negative charge. Neutrons have no charge. The only exception is Hydrogen-1, which has one proton and one electron but no neutrons. Also, a positive hydrogen ion has only one proton and no electrons. All other atoms usually have at least one proton, one neutron, and one electron.
Electrons are the smallest of the three atomic particles. They are so tiny that their mass and size are hard to measure with today's technology. Protons and neutrons are similar in size and weight to each other.
Most atoms have a neutral charge overall. This is because they have the same number of protons (positive) and electrons (negative), so their charges balance out to zero. However, ions are atoms that have a different number of electrons, so they can have a positive or negative charge. Protons and neutrons are made of even smaller particles called quarks. There are two types: up quarks and down quarks. A proton has two up quarks and one down quark, while a neutron has two down quarks and one up quark.
The Nucleus: Atom's Center
The nucleus is right in the middle of an atom. It's made of protons and neutrons. Normally, things with the same charge push away from each other. So, for a long time, scientists wondered how the positively charged protons in the nucleus stayed together. They found a particle called a gluon. Gluons act like "atomic glue," holding the protons together using a very strong force called the strong nuclear force. This force also holds the quarks together inside the protons and neutrons.
The number of neutrons compared to protons in a nucleus affects whether it is stable or undergoes radioactive decay. If there are too many neutrons or protons, the atom tries to become stable by getting rid of the extra particles. It does this by releasing radiation in the form of alpha, beta, or gamma decay.
Nuclei can also change in other ways. Nuclear fission happens when a nucleus splits into two smaller nuclei, releasing a huge amount of stored energy. This energy release is why nuclear fission is used to make bombs and generate electricity in nuclear power plants.
Another way nuclei can change is through nuclear fusion. This is when two nuclei join together, or fuse, to create a heavier nucleus. This process needs extreme amounts of energy to overcome the push between the protons. Such high energies are common in stars like our Sun, which uses fusion to create its energy by combining hydrogen atoms.
Electrons: The Outer Cloud
Electrons travel around the nucleus in what's called the atom's electron cloud. They are pulled towards the nucleus by the electromagnetic force because electrons have a negative charge and the nucleus has a positive charge.
Electrons are arranged in different layers or electron shells around the nucleus. In most atoms, the first shell holds two electrons, and the shells after that hold eight. The further an electron is from the nucleus, the weaker the nucleus's pull on it. This is why larger atoms, with more electrons, can react more easily with other atoms. Sometimes, the nucleus's electromagnetism isn't strong enough to hold onto its outer electrons, and the atom can lose them to smaller atoms with stronger pulls.
When Atoms Change: Radioactive Decay
Some elements and many isotopes have an unstable nucleus. This means the nucleus might be too big to hold itself together, or it has too many protons or neutrons. When this happens, the nucleus gets rid of the extra mass or particles by releasing radiation. An atom that does this is called radioactive. Unstable atoms keep being radioactive until they lose enough mass or particles to become stable. All atoms with an atomic number above 82 (meaning more than 82 protons, like lead) are radioactive.
There are three main types of radioactive decay: alpha, beta, and gamma.
- Alpha decay is when an atom shoots out a particle made of two protons and two neutrons. This is basically a helium nucleus. The atom then becomes a different element with an atomic number two less than before. For example, if a beryllium atom (atomic number 4) goes through alpha decay, it becomes helium (atomic number 2). Alpha decay happens when an atom is too big and needs to lose some mass.
- Beta decay is when a neutron changes into a proton, or a proton changes into a neutron. If a neutron turns into a proton, the atom shoots out an electron. If a proton turns into a neutron, it shoots out a positron (which is like an electron but with a positive charge). The result is an element with an atomic number one higher or one lower than before. Beta decay happens when an atom has too many protons or too many neutrons.
- Gamma decay is when an atom releases a gamma ray, which is a type of energy wave. This usually happens after a nucleus has already gone through alpha or beta decay. There is no change in the atom's mass or atomic number, only in the energy stored inside the nucleus.
Every radioactive element or isotope has a half-life. This is the time it takes for half of any sample of those atoms to decay and change into a different, stable isotope or element. Large atoms or isotopes with a big difference between their protons and neutrons will have a long half-life because they need to lose more particles to become stable.
Marie Curie discovered the first form of radiation. She found the element and named it radium. She was also the first woman to win a Nobel Prize.
Frederick Soddy did an experiment to see what happens as radium decays. He put a sample in a light bulb and waited. Suddenly, helium (with two protons and two neutrons) appeared in the bulb. From this, he discovered that this type of radiation has a positive charge.
James Chadwick discovered the neutron. He noticed that the atomic number of elements was lower than the total atomic mass of the atom. He realized that electrons couldn't cause this extra mass because they are so light. So, he concluded that neutrons must exist.
Enrico Fermi used neutrons to bombard uranium. He found that uranium decayed much faster than usual and produced many alpha and beta particles. He thought the uranium changed into a new element he called hesperium.
Otto Hahn and Fritz Strassmann repeated Fermi's experiment. They found two new things Fermi hadn't seen. They discovered that by using many neutrons, the nucleus of the atom would split, releasing a lot of heat energy. They also found that the products of uranium fission were already known elements like thorium, palladium, radium, radon, and lead.
Fermi then noticed that when one uranium atom split, it released more neutrons, which could then split other atoms. This created a chain reaction. He realized this process, called nuclear fission, could create huge amounts of heat energy. This discovery eventually led to the development of the first nuclear bomb, code-named 'Trinity'.
Images for kids
-
Atoms and molecules as drawn by John Dalton in 1808.
-
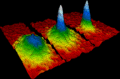
A picture showing how a Bose–Einstein condensate forms.
-
A special microscope image showing individual atoms on a gold surface.
-
Ernest Rutherford, a key scientist in understanding the atom's nucleus.
-
This diagram shows how protons, which have positive charges, push away from each other. This is a big challenge in nuclear fusion.
See also
 In Spanish: Átomo para niños
In Spanish: Átomo para niños