Palladium facts for kids
Palladium is a special chemical element. Think of it as one of the basic building blocks that make up everything around us! Its chemical symbol is Pd, and its atomic number is 46. This number tells us how many protons are in its atoms.
Palladium is a beautiful, shiny metal that looks silver-white. In the world of chemistry, it belongs to a group of metals called transition metals. It's also part of an even more exclusive club known as the platinum group metals, which includes other valuable metals like platinum itself.
Palladium is quite similar to platinum in how it behaves. We get palladium by taking it out of certain ores, which are rocks that contain valuable metals. These ores often also contain copper and nickel. Palladium is super useful! Its main jobs are acting as a catalyst (which helps chemical reactions happen faster without being used up itself) and being used to make beautiful jewellery.
| Periodic table | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
Contents
What is Palladium?
A Special Metal Element
Palladium is a natural element found on Earth. It's known for being very strong and not reacting easily with other chemicals. This makes it a "noble metal," meaning it resists corrosion and tarnishing. It's also quite rare, which adds to its value.
Where Does Palladium Come From?
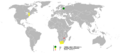
Most of the world's palladium comes from Russia and South Africa. It's usually found mixed with other metals like platinum, nickel, and copper. Miners dig up these ores, and then special processes are used to separate the palladium from the other metals. It's a complex process to get pure palladium!
Uses of Palladium
In Cars: Catalytic Converters
One of the biggest uses for palladium is in catalytic converters in cars. These devices help clean up the exhaust fumes from engines. Palladium acts as a catalyst, changing harmful gases like carbon monoxide and nitrogen oxides into less harmful ones like carbon dioxide and water vapor. This helps protect our air!
For Jewellery
Because palladium is shiny, strong, and doesn't tarnish, it's a popular choice for making jewellery. It's often used in white gold alloys, which are mixtures of gold and other metals. Palladium can also be used on its own to create rings, necklaces, and other beautiful pieces. It's a great alternative to platinum for those who want a similar look.
Other Important Uses
Palladium has many other uses too. It's used in dentistry for fillings and crowns. You can also find it in electronics, like in computer parts and mobile phones. Sometimes, it's even used in medicine and in hydrogen purification, which is a process to make hydrogen gas very pure.
History of Palladium
Discovery of Palladium
Palladium was discovered in 1803 by a British chemist named William Hyde Wollaston. He found it while working with platinum ore. He named it after the asteroid Pallas, which had been discovered just a few years earlier. At first, people were unsure if it was a new element, but Wollaston proved it was unique.
Early Uses
In its early days, palladium was mostly used in dentistry and for making special instruments. As time went on and its unique properties became better understood, its uses expanded. The demand for palladium grew a lot in the late 20th century, especially with the rise of catalytic converters in cars.
Images for kids
-
Cross section of a metal-core catalytic converter
See also
 In Spanish: Paladio para niños
In Spanish: Paladio para niños
 | Mary Eliza Mahoney |
 | Susie King Taylor |
 | Ida Gray |
 | Eliza Ann Grier |