Nobelium facts for kids
Nobelium is a synthetic element, which means it doesn't naturally exist on Earth. Scientists create it in special laboratories. Its chemical symbol is No, and its atomic number is 102. This number tells us how many protons are in the center of each nobelium atom.
Nobelium is a radioactive metal. This means its atoms are unstable and slowly break down, releasing energy. It's the tenth transuranium element, which are elements with an atomic number higher than 92 (Uranium). Nobelium is also part of the actinide series on the periodic table, a group of similar heavy elements. Scientists know of twelve different forms of nobelium, called isotopes, but they all last for only a very short time before decaying.
Contents
Discovering Nobelium
Scientists first claimed to have discovered Nobelium in 1957. A team from Sweden said they found it. They named it "Nobelium" after Alfred Nobel, who created the famous Nobel Prizes. However, other scientists couldn't repeat their experiment. This meant the first discovery was not confirmed.
Who Really Found It?
The real discovery of Nobelium happened a few years later. In 1966, a team of scientists in Dubna, Russia, successfully created Nobelium. They used a special machine called a particle accelerator to smash atoms together. They made an isotope called Nobelium-254. Soon after, American scientists at the Lawrence Berkeley National Laboratory in California also made Nobelium. They confirmed the Russian team's findings.
What is Nobelium Like?
Nobelium is a very rare and unstable element. Because it's so hard to make and decays quickly, scientists can't study it easily. They can only make tiny amounts, often just a few atoms at a time.
Chemical Properties
Scientists predict that Nobelium is a soft, silvery metal. It's expected to be quite reactive, like other metals in the actinide series. One interesting thing about Nobelium is that it often forms chemical compounds where it has a charge of +2. This is different from many other actinides, which usually have a charge of +3.
Radioactive Isotopes
All isotopes of Nobelium are radioactive. The most stable isotope is Nobelium-259, but even that only lasts for about 58 minutes. Other isotopes decay much faster, sometimes in just a few seconds or even milliseconds. Because it decays so quickly, Nobelium has no practical uses outside of scientific research. Scientists study it to better understand the properties of very heavy elements and how atoms behave.
Where is Nobelium Found?
Nobelium is not found anywhere on Earth naturally. It can only be made in laboratories. Scientists create it by bombarding lighter elements with high-energy particles. For example, they might shoot calcium ions at a target made of curium. This process creates a few atoms of Nobelium.
Images for kids
-
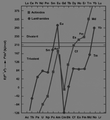
Energy required to promote an f electron to the d subshell for the f-block lanthanides and actinides. Above around 210 kJ/mol, this energy is too high to be provided for by the greater crystal energy of the trivalent state and thus einsteinium, fermium, and mendelevium form divalent metals like the lanthanides europium and ytterbium. Nobelium is also expected to form a divalent metal, but this has not yet been confirmed.
See also
 In Spanish: Nobelio para niños
In Spanish: Nobelio para niños