Isotope facts for kids
Imagine atoms as tiny building blocks of everything around us. A chemical element like oxygen or carbon is made of a specific type of atom. But did you know that atoms of the same element can come in slightly different versions? These different versions are called isotopes.
Isotopes of an element are like siblings in a family. They are part of the same family (the same element), but they have a small difference. This difference is in their weight. All isotopes of an element have the same number of protons. This is what makes them that specific element. They also have the same number of electrons. But they have a different number of neutrons. Because neutrons have mass, this means isotopes of the same element have different masses and weights.
Contents
What are Isotopes?
Every atom has a center called the nucleus. Inside the nucleus are two types of tiny particles: protons and neutrons. Around the nucleus, even smaller particles called electrons fly around.
- Protons have a positive electric charge. The number of protons in an atom decides what element it is. For example, all carbon atoms have 6 protons.
- Neutrons have no electric charge. They are neutral.
- Electrons have a negative electric charge. In a normal, neutral atom, the number of electrons is the same as the number of protons.
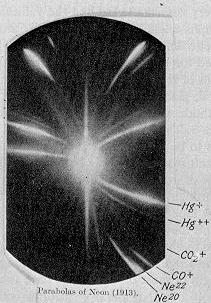
Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. Because they have the same number of protons, they are found in the same spot on the periodic table. This is why they are called "isotopes," which means "at the same place."
How Isotopes Are Different
Even though isotopes of the same element are very similar, their different number of neutrons makes them behave slightly differently.
- Mass and Weight: Since neutrons add to the atom's mass, isotopes with more neutrons are heavier. Think of it like adding more bricks to a small building.
- Chemical Behavior: An atom's chemical behavior is mostly decided by its electrons. Since isotopes of the same element have the same number of electrons, they act almost the same chemically. However, heavier isotopes can sometimes react a little slower than lighter ones. This difference is usually very small, except for very light elements like hydrogen.
Why Neutrons Matter
Protons are all positively charged, so they naturally push away from each other. Neutrons act like a glue inside the nucleus. They help hold the protons together, stopping them from flying apart. Without neutrons, it would be very hard for two or more protons to stick together in a nucleus. As an element gets more protons, it needs even more neutrons to keep its nucleus stable.
Types of Isotopes
Some elements in nature have only one type of isotope. For example, fluorine (19F) only has one stable isotope. Other elements can have many isotopes. Tin, for instance, has ten different stable isotopes!
Isotopes can be divided into two main groups:
- Stable Isotopes: These isotopes do not change over time. They stay the same.
- Radioactive Isotopes: These isotopes are unstable. They slowly break down and release energy and particles. This process is called radiation.
Hydrogen's Isotopes
Hydrogen is the simplest element, and it has three common isotopes:
- Protium (1H): This is the most common type of hydrogen. It has one proton and no neutrons.
- Deuterium (2H): This hydrogen atom has one proton and one neutron. It's sometimes called "heavy hydrogen." Protium and deuterium are both stable.
- Tritium (3H): This hydrogen atom has one proton and two neutrons. Tritium is a radioactive isotope.
Heavy Elements and Radioactivity
The very heaviest elements on the periodic table are all radioactive. Elements like radon, thorium, and uranium are examples. Their nuclei are so packed with protons and neutrons that the nuclear forces struggle to hold them all together. This makes them unstable and causes them to break down over time.
See also
 In Spanish: Isótopo para niños
In Spanish: Isótopo para niños

