Tin facts for kids
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tin | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allotropes | silvery-white, β (beta); gray, α (alpha) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery-white (beta, β) or gray (alpha, α) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar, std(Sn) | 118.710(7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tin in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 50 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 14 (carbon group) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | p | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Kr] 4d10 5s2 5p2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 18, 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 505.08 K (231.93 °C, 449.47 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 2875 K (2602 °C, 4716 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | white, β: 7.265 g/cm3 gray, α: 5.769 g/cm3 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 6.99 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | white, β: 7.03 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | white, β: 296.1 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | white, β: 27.112 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −4, −3, −2, −1, +1, +2, +3, +4 (an amphoteric oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.96 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 140 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 139±4 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 217 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectral lines of tin | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
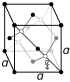
| Crystal structure | tetragonal
white (β) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | face-centered diamond-cubic
gray (α) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 2730 m/s (at r.t.) (rolled) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 22.0 µm/(m⋅K) (at 25 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 66.8 W/(m⋅K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 115 nΩ⋅m (at 0 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | gray: diamagnetic white (β): paramagnetic |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | (white) +3.1·10−6 cm3/mol (298 K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 50 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 18 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 58 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 50–440 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-31-5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | around 3500 BC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symbol | "Sn": from Latin stannum | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Main isotopes of tin | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Tin is a chemical element with the symbol Sn and atomic number 50. The symbol Sn comes from its Latin name, stannum. Tin is a silvery-gray metal that is so soft you can cut it with little force. You can even bend a bar of tin by hand.
When you bend a piece of tin, it makes a strange cracking sound called the "tin cry." This sound comes from the tiny crystals inside the metal rubbing against each other as they move.
Tin is a post-transition metal in group 14 of the periodic table. It is found in nature mainly in a mineral called cassiterite. Tin is similar to its neighbors on the periodic table, germanium and lead. It is the 49th most common element in the Earth's crust.
One of the first important uses for tin was to make bronze, a strong metal alloy made by mixing tin with copper. Today, tin is used for many things, from coating steel cans to protect food, to making the solder that holds electronic circuits together.
Contents
Characteristics
Physical Properties
Tin is a soft, silvery-white metal that can be easily shaped without breaking. This property is called being malleable. As mentioned before, it makes a unique "tin cry" sound when bent. This is a special trait it shares with a few other metals like indium and cadmium.
Tin has a low melting point for a metal, at about 232 °C (450 °F). This makes it easy to melt and mix with other metals to form alloys.
Two Forms of Tin
Tin can exist in two main forms, called allotropes.
- β-tin (Beta-tin), or white tin, is the normal metallic form we see at room temperature. It is shiny and easy to work with.
- α-tin (Alpha-tin), or gray tin, is a non-metallic powder. This form appears when tin gets very cold, below 13.2 °C (55.8 °F).
When white tin gets too cold, it can slowly turn into gray tin powder. This change is called "tin pest" or "tin disease" because it can cause tin objects to crumble and fall apart. There is a legend that during Napoleon's invasion of Russia in 1812, the extreme cold caused the tin buttons on his soldiers' uniforms to crumble away, which added to their problems.
To prevent tin pest, small amounts of other metals like antimony or bismuth are often added to tin. This makes the tin stronger and more durable, especially in the cold.
A Special Element: Isotopes
Isotopes are different versions of an element's atom that have a different number of neutrons. Tin is special because it has ten stable isotopes, which is more than any other element in the periodic table.
Scientists believe this is because tin's atomic number is 50. In nuclear physics, 50 is a "magic number" of protons, which makes the atom's nucleus unusually stable.
History of Tin
People started using tin around 3000 BC, at the beginning of the Bronze Age. Early metalworkers discovered that if they mixed a small amount of tin (about 12%) with copper, they could create a new metal called bronze.
Bronze was much harder and stronger than pure copper. It was also easier to melt and cast into complex shapes like tools, weapons, and armor. This discovery was a major step forward for human technology.
Since tin was not very common, a large trade network was created to bring tin from faraway mines to the civilizations that needed it.
Later, after 600 BC, people learned how to produce pure tin. Another important tin alloy is pewter, which is about 90% tin. Pewter was used for centuries to make plates, cups, and other tableware because it was easy to shape and didn't rust.
Where is Tin Found?
Tin is not found as a pure metal in nature. It is extracted from ores, with the most important one being cassiterite (tin oxide, SnO2). Cassiterite is a hard, heavy mineral that is often found in or near granite rock.
Over millions of years, wind and rain wear down the rock, and the heavy cassiterite gets washed into rivers and streams. It settles on the riverbeds in deposits called placer deposits. About 80% of the world's tin is mined from these deposits.
Today, the largest tin-producing countries are China, Indonesia, Peru, Bolivia, and Brazil.
What is Tin Used For?
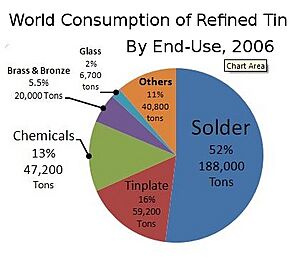
Tin is a very useful metal with many modern applications. In 2018, almost half of all tin was used to make solder.
Solder
Solder is a metal alloy that is melted to join metal pieces together, like a type of metal glue. For a long time, solder was made from tin and lead. It is essential for making electric circuits, as it connects all the tiny components on a circuit board.
Because lead can be harmful to people's health and the environment, most countries now require lead-free solder. Modern lead-free solders are usually made of mostly tin, with small amounts of copper and silver.
Tin Plating and Cans
Tin is excellent at preventing rust. Because of this, it is often used to coat other metals like steel. The "tin cans" we use for food are actually steel cans with a very thin layer of tin on the inside and outside. This tin plating protects the steel from rusting and keeps the food inside safe to eat.
Copper pots and pans are also often lined with tin. This is because copper can react with acidic foods, but the tin lining creates a safe barrier.
Important Alloys
An alloy is a mixture of two or more metals. Tin is a key ingredient in several important alloys.
- Bronze is an alloy of copper and tin. It is used to make statues, bells, and medals.
- Pewter is an alloy that is mostly tin, with small amounts of copper and antimony. It is used to make decorative items like plates, mugs, and figurines.
- Babbitt metal is a soft alloy of tin used to make bearings for machines, helping parts move smoothly with little friction.
Other Cool Uses
- Making Glass: Most of the flat window glass we use today is made using a method called the Pilkington process. In this process, melted glass is floated on top of a large pool of molten tin. Because the tin is perfectly flat, the glass cools into a smooth, flawless sheet.
- Touch Screens: A compound called Indium tin oxide is both transparent and electrically conductive. It is used to make the clear touch screens on smartphones, tablets, and TVs.
- Toothpaste: Some toothpastes contain a compound called stannous fluoride. It helps fight cavities and prevent gum problems like gingivitis.
Safety
Tin metal is considered safe for humans to handle. This is why it has been used for centuries in food containers and kitchenware.
However, some special man-made tin compounds, known as organotin compounds, can be harmful if not handled properly. These chemicals were once used in paint for ships to stop barnacles from growing on them. But scientists discovered they were harming sea life, so their use is now heavily restricted around the world. People who work with these chemicals follow strict safety rules to protect themselves and the environment.
See also
 In Spanish: Estaño para niños
In Spanish: Estaño para niños
 | Percy Lavon Julian |
 | Katherine Johnson |
 | George Washington Carver |
 | Annie Easley |