Helium facts for kids
 |
||||||||||||||||
| Helium | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈhiːliəm/ |
|||||||||||||||
| Appearance | colorless gas, exhibiting a red-orange glow when placed in an electric field | |||||||||||||||
| Standard atomic weight Ar, std(He) | 4.002602(2) | |||||||||||||||
| Helium in the periodic table | ||||||||||||||||
|
||||||||||||||||
| Atomic number (Z) | 2 | |||||||||||||||
| Group | group 18 (noble gases) | |||||||||||||||
| Period | period 1 | |||||||||||||||
| Block | p | |||||||||||||||
| Electron configuration | 1s2 | |||||||||||||||
| Electrons per shell | 2 | |||||||||||||||
| Physical properties | ||||||||||||||||
| Phase at STP | gas | |||||||||||||||
| Melting point | 0.95 K (−272.20 °C, −457.96 °F) (at 2.5 MPa) | |||||||||||||||
| Boiling point | 4.222 K (−268.928 °C, −452.070 °F) | |||||||||||||||
| Density (at STP) | 0.1786 g/L | |||||||||||||||
| when liquid (at m.p.) | 0.145 g/cm3 | |||||||||||||||
| when liquid (at b.p.) | 0.125 g/cm3 | |||||||||||||||
| Triple point | 2.177 K, 5.043 kPa | |||||||||||||||
| Critical point | 5.1953 K, 0.22746 MPa | |||||||||||||||
| Heat of fusion | 0.0138 kJ/mol | |||||||||||||||
| Heat of vaporization | 0.0829 kJ/mol | |||||||||||||||
| Molar heat capacity | 20.78 J/(mol·K) | |||||||||||||||
Vapor pressure (defined by ITS-90)
|
||||||||||||||||
| Atomic properties | ||||||||||||||||
| Oxidation states | 0 | |||||||||||||||
| Electronegativity | Pauling scale: no data | |||||||||||||||
| Ionization energies |
|
|||||||||||||||
| Covalent radius | 28 pm | |||||||||||||||
| Van der Waals radius | 140 pm | |||||||||||||||
| Spectral lines of helium | ||||||||||||||||
| Other properties | ||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||
| Crystal structure | hexagonal close-packed (hcp) | |||||||||||||||
| Speed of sound | 972 m/s | |||||||||||||||
| Thermal conductivity | 0.1513 W/(m⋅K) | |||||||||||||||
| Magnetic ordering | diamagnetic | |||||||||||||||
| Molar magnetic susceptibility | −1.88·10−6 cm3/mol (298 K) | |||||||||||||||
| CAS Number | 7440-59-7 | |||||||||||||||
| History | ||||||||||||||||
| Naming | after Helios, Greek Titan of the Sun | |||||||||||||||
| Discovery | Pierre Janssen, Norman Lockyer (1868) | |||||||||||||||
| First isolation | William Ramsay, Per Teodor Cleve, Abraham Langlet (1895) | |||||||||||||||
| Main isotopes of helium | ||||||||||||||||
|
||||||||||||||||

Helium is a special kind of building block for everything around us, called a chemical element. Its short name is He. It has an atomic number of 2, which means it has 2 protons in its center.
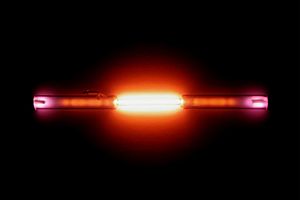
Helium is known as a noble gas. This means it doesn't usually mix with other chemicals to form new things. It has the lowest boiling point of all elements, meaning it stays a gas even in extremely cold conditions. After hydrogen, helium is the second most common element in the universe. It has no color or smell. However, if you put helium in an electric field, it glows with a red-orange light. Scientists first found helium in 1868 by looking at light from the Sun, even before they found it on Earth.
Helium is used to fill balloons and airships because it is much lighter than air. It also doesn't burn, which makes it very safe for these uses. You can find it in some types of light bulbs too. People sometimes breathe in helium for fun because it makes their voices sound higher. But this can be dangerous! Breathing too much helium means you aren't getting enough normal air, which can lead to a lack of oxygen (called hypoxia). This can hurt or even kill you. It can also cause long-term damage to your vocal cords.
On the Sun and other stars, helium is made through a process called nuclear fusion. This is when four hydrogen atoms join together to create one helium atom. On Earth, helium is made naturally when heavy elements like thorium and uranium slowly break down (this is called radioactive decay). The tiny particles released during this process are actually helium atoms.
Contents
Discovery of Helium
Helium was first spotted by a French astronomer named Pierre Janssen on August 18, 1868. He saw a bright yellow line in the light coming from the Sun during a solar eclipse. At first, he thought it was sodium. In the same year, an English astronomer, Norman Lockyer, also saw this line. He realized it was caused by a brand new element. Lockyer and another English chemist, Edward Frankland, decided to name this new element helium. They got the name from the Greek word for the Sun, helios.
What Makes Helium Special?
Helium is the second least reactive noble gas, right after neon. This means it almost never reacts with other chemicals. It's also the second least reactive of all known elements. Helium is usually found as single atoms and doesn't easily dissolve in water.
How We Use Helium
Helium has many important uses because it doesn't react with other things:
- It's used as a "shielding gas" when making things like silicon for computer chips or strong metals like titanium. It protects these materials from reacting with air.
- It's also used in arc welding to keep the metal safe from air.
- For deep underwater diving, helium is mixed with oxygen and other gases. This mixture helps divers avoid a dizzy feeling called nitrogen narcosis.
- Helium helps to cool down hydrogen and oxygen to make rocket fuel. It's also used to clean out the fuel lines of rockets before they launch.
- Some nuclear reactors use helium to cool them down.
- You can even find helium inside some hard disk drives to help them work better.
- At very low temperatures, helium is used in a field called cryogenics. This is about studying and working with extremely cold things.
Where Does Our Helium Come From?
Helium is becoming quite rare on Earth. If it escapes into the air, it's so light that it floats right off the planet. Unlike hydrogen, which can combine with oxygen to form water, helium doesn't react. It just stays a gas and drifts away.
For many years, the USA collected and stored helium in a special reserve. Most American helium comes from wells in the Great Plains area. Today, more helium is supplied by Qatar than by the USA.
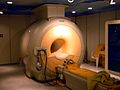
Many scientists and organizations have warned about the limited supply of helium. They've asked governments to save and protect helium because it's so unique and important. For researchers, helium is super important because it's needed to create very low temperatures. Liquid helium is used to cool certain metals to extremely cold temperatures. This allows them to become superconducting, which means electricity can flow through them with no resistance. This is used in powerful magnets for magnetic resonance imaging (MRI) machines, which doctors use to see inside the body.
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrogen & alkali metals |
Alkaline earth metals | Triels | Tetrels | Pnictogens | Chalcogens | Halogens | Noble gases |
||||||||||||
| Period |
|||||||||||||||||||
| 2 | |||||||||||||||||||
| 3 | |||||||||||||||||||
| 4 | |||||||||||||||||||
| 5 | |||||||||||||||||||
| 6 | |||||||||||||||||||
| 7 | |||||||||||||||||||
Primordial From decay Synthetic Border shows natural occurrence of the element
- Ca: 40.078 — Abridged value (uncertainty omitted here)
- Po: [209] — mass number of the most stable isotope
Images for kids
-
Sir William Ramsay, who discovered helium on Earth.
-
A historical marker showing where a lot of helium was found in Dexter, Kansas.
-
Liquid helium. This helium is so cold it's become a superfluid, meaning it can flow without any friction.
See also
 In Spanish: Helio para niños
In Spanish: Helio para niños