Carbon facts for kids

Graphite (left) and diamond (right), two allotropes of carbon
|
||||||||||||||||||||||||||
| Carbon | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allotropes | graphite, diamond and more (see Allotropes of carbon) | |||||||||||||||||||||||||
| Appearance | graphite: black diamond: clear |
|||||||||||||||||||||||||
| Standard atomic weight Ar, std(C) | [12.0096, 12.0116] conventional: 12.011 | |||||||||||||||||||||||||
| Carbon in the periodic table | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Atomic number (Z) | 6 | |||||||||||||||||||||||||
| Group | group 14 (carbon group) | |||||||||||||||||||||||||
| Period | period 2 | |||||||||||||||||||||||||
| Block | p | |||||||||||||||||||||||||
| Electron configuration | [He] 2s2 2p2 | |||||||||||||||||||||||||
| Electrons per shell | 2, 4 | |||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||
| Phase at STP | solid | |||||||||||||||||||||||||
| Sublimation point | 3915 K (3642 °C, 6588 °F) | |||||||||||||||||||||||||
| Density (near r.t.) | amorphous: 1.8–2.1 g/cm3 graphite: 2.267 g/cm3 diamond: 3.515 g/cm3 |
|||||||||||||||||||||||||
| Triple point | 4600 K, 10,800 kPa | |||||||||||||||||||||||||
| Heat of fusion | graphite: 117 kJ/mol | |||||||||||||||||||||||||
| Molar heat capacity | graphite: 8.517 J/(mol·K) diamond: 6.155 J/(mol·K) |
|||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||
| Oxidation states | −4, −3, −2, −1, 0, +1, +2, +3, +4 (a mildly acidic oxide) | |||||||||||||||||||||||||
| Electronegativity | Pauling scale: 2.55 | |||||||||||||||||||||||||
| Ionization energies |
|
|||||||||||||||||||||||||
| Covalent radius | sp3: 77 pm sp2: 73 pm sp: 69 pm |
|||||||||||||||||||||||||
| Van der Waals radius | 170 pm | |||||||||||||||||||||||||
| Spectral lines of carbon | ||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||||||||||||
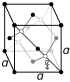
| Crystal structure | graphite: simple hexagonal
(black) |
|||||||||||||||||||||||||
| Crystal structure | diamond: face-centered diamond-cubic
(clear) |
|||||||||||||||||||||||||
| Speed of sound thin rod | diamond: 18,350 m/s (at 20 °C) | |||||||||||||||||||||||||
| Thermal expansion | diamond: 0.8 µm/(m⋅K) (at 25 °C) | |||||||||||||||||||||||||
| Thermal conductivity | graphite: 119–165 W/(m⋅K) diamond: 900–2300 W/(m⋅K) |
|||||||||||||||||||||||||
| Electrical resistivity | graphite: 7.837 µΩ⋅m | |||||||||||||||||||||||||
| Magnetic ordering | diamagnetic | |||||||||||||||||||||||||
| Molar magnetic susceptibility | −5.9·10−6 (graph.) cm3/mol | |||||||||||||||||||||||||
| Young's modulus | diamond: 1050 GPa | |||||||||||||||||||||||||
| Shear modulus | diamond: 478 GPa | |||||||||||||||||||||||||
| Bulk modulus | diamond: 442 GPa | |||||||||||||||||||||||||
| Poisson ratio | diamond: 0.1 | |||||||||||||||||||||||||
| Mohs hardness | graphite: 1–2 diamond: 10 |
|||||||||||||||||||||||||
| CAS Number | 7440-44-0 | |||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||
| Discovery | Egyptians and Sumerians (3750 BCE) | |||||||||||||||||||||||||
| Recognized as an element by | Antoine Lavoisier (1789) | |||||||||||||||||||||||||
| Main isotopes of carbon | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
Carbon is a super important chemical element that all known life on Earth needs! Its chemical symbol is C. Carbon has an atomic number of 6 and an atomic mass of 12. It's a nonmetal, which means it's not shiny like a metal and doesn't conduct electricity well.
When iron is mixed with carbon, it creates strong steel. Carbon, often found as coal, is also a very important fuel that helps power many things.
Contents
The Amazing Chemistry of Carbon
A whole branch of science, called organic chemistry, is all about carbon and its many compounds. Carbon can form a huge number of different compounds. For example, hydrocarbons are molecules made only of carbon and hydrogen. Many common fuels like methane and propane are hydrocarbons. Lots of things we use every day are organic compounds!
Carbon, along with hydrogen, nitrogen, oxygen, sulfur, and phosphorus, makes up most living things on Earth. You can see more about these in the list of biologically important elements. Carbon is special because it can form strong bonds with itself and with other elements. This is why living things are often called "carbon-based."
Each carbon atom can make four single covalent bonds. These bonds let carbon create long chain-shaped molecules, called polymers. Think of plastics – they are polymers made possible by carbon!
Where the Name Carbon Comes From
The name "carbon" comes from the Latin word carbo, which means charcoal. In many languages around the world, the words for carbon, coal, and charcoal are very similar or even the same.
Different Forms of Carbon
Carbon can be found in nature in three main forms, called allotropes. These are diamond, graphite, and fullerenes.
- Graphite is very soft. It's what's mixed with clay to make the "lead" in pencils. The carbon atoms in graphite are arranged in rings that stack on top of each other and can slide easily.
- Diamonds are the hardest natural mineral known! They are incredibly strong.
- Fullerenes are shaped like a tiny soccer ball. Scientists are very interested in studying them.
There's also a special, man-made form of carbon called a carbon nanotube. These are tiny, tube-shaped structures that are incredibly strong. Because they are so hard, carbon nanotubes might be used in things like armor or in new technologies like nanotechnology.
There are over 10 million known carbon compounds! That's a lot!

How Radiocarbon Dating Works
Scientists use a special radioactive form of carbon, called carbon-14, to figure out how old ancient objects are. This method is called radiocarbon dating.
While something is alive and taking in carbon from the environment, the amount of carbon-14 inside it stays the same. But once it dies or stops taking in carbon, the carbon-14 slowly starts to disappear. This happens at a steady rate. The half-life of carbon-14 is 5,730 years. This means it takes 5,730 years for half of the carbon-14 to go away. By measuring how much carbon-14 is left in an object, scientists can tell how old it is!
Where Can We Find Carbon?
Carbon is found all over the universe! It was first created inside old stars. Carbon is the fourth most common element in our own sun. The atmospheres of planets like Venus and Mars are mostly made of carbon dioxide.
Carbon is also super important for the human body and all other living things. It's the second most common element in the human body, making up about 23% of our body weight! It's a key part of many important molecules that life needs to work.
On Earth, most carbon is found as coal. Graphite can be found in many places, especially in desert areas like Sri Lanka, Madagascar, and Russia. Diamonds are much rarer and are mostly found in Africa. You can even find carbon in some meteorites that fall to Earth!
Related Pages
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrogen & alkali metals |
Alkaline earth metals | Triels | Tetrels | Pnictogens | Chalcogens | Halogens | Noble gases |
||||||||||||
| Period |
|||||||||||||||||||
| 2 | |||||||||||||||||||
| 3 | |||||||||||||||||||
| 4 | |||||||||||||||||||
| 5 | |||||||||||||||||||
| 6 | |||||||||||||||||||
| 7 | |||||||||||||||||||
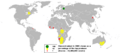
Primordial From decay Synthetic Border shows natural occurrence of the element
- Ca: 40.078 — Abridged value (uncertainty omitted here)
- Po: [209] — mass number of the most stable isotope
Images for kids
-
Comet C/2014 Q2 (Lovejoy) surrounded by glowing carbon vapor
-
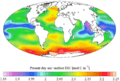
"Present day" (1990s) sea surface dissolved inorganic carbon concentration (from the GLODAP climatology)
-
Correlation between the carbon cycle and formation of organic compounds. In plants, carbon dioxide formed by carbon fixation can join with water in photosynthesis (green) to form organic compounds, which can be used and further converted by both plants and animals.
-
Antoine Lavoisier in his youth
-
Pencil leads for mechanical pencils are made of graphite (often mixed with a clay or synthetic binder).
-
Sticks of vine and compressed charcoal
See also
 In Spanish: Carbono para niños
In Spanish: Carbono para niños