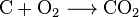Carbon dioxide facts for kids
Carbon dioxide (CO2) is a chemical compound that is a gas at room temperature. It is made of one carbon atom and two oxygen atoms.
People and animals release carbon dioxide when they breathe out. Also, when anything organic burns (like wood in a fire), it creates carbon dioxide. Plants use carbon dioxide to make their own food through a process called photosynthesis.
A Scottish scientist named Joseph Black first studied carbon dioxide in the 1750s.
Carbon dioxide is also a greenhouse gas. Greenhouse gases trap heat on our planet, Earth. This trapping of heat can change our climate and weather, which is known as climate change. Greenhouse gases are a main cause of global warming, which is the rise in Earth's surface temperature.
Contents
How Carbon Dioxide is Used by Living Things
Carbon dioxide is a natural part of how living things get energy. When organisms like plants, animals, fungi, and some bacteria break down sugars, fats, and other things with oxygen, they produce carbon dioxide. This process is called cellular respiration.
In animals, carbon dioxide travels in the blood from the body's tissues to the lungs, where it is breathed out. Plants, on the other hand, take in carbon dioxide from the air to use for photosynthesis.
What is Dry Ice?
Dry ice is the solid form of carbon dioxide. It is extremely cold, below -78.5 °C (-109.3 °F). Dry ice does not exist naturally on Earth; it is made by people. It has no color.
People use dry ice to keep things cold, to make drinks fizzy, and even to create special effects. For example, it's often used in theaters to make fog or smoke.
When dry ice gets warmer, it doesn't melt into a liquid. Instead, it changes directly from a solid to a gas. This process is called sublimation. The gas that comes off dry ice is so cold that it cools the water vapor in the air, creating a thick white fog.
Important Safety Note: The gas from dry ice can cause suffocation if there isn't enough fresh air. Always be careful and get help from an adult when using dry ice.
How Carbon Dioxide is Made
Carbon dioxide is made in many ways, both naturally and by humans.
Natural and Everyday Ways
- Breathing: As we learned, people and animals breathe out carbon dioxide.
- Burning: When things like wood, natural gas, gasoline, or coal burn, they release carbon dioxide. This is called combustion. For example, burning methane (a main part of natural gas) with oxygen makes carbon dioxide and water:
: 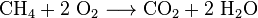
- Fermentation: Yeast uses sugar to make carbon dioxide and ethanol (alcohol). This process is used to make wines, beers, and even bioethanol.
: 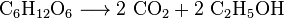
- Plants: Plants absorb carbon dioxide from the air and use it with water to create carbohydrates (their food) and oxygen.
: 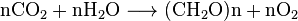
Industrial Ways
- Chemical Reactions: Carbon dioxide is made when most acids react with most metal carbonates (like limestone or chalk). For example, mixing hydrochloric acid with limestone creates carbon dioxide.
- Making Lime: When quicklime (a chemical used in many industries) is made by heating limestone, carbon dioxide is also produced.
- Steel Mills: In steel mills, carbon dioxide is made when iron is separated from its ores using coke.
Simple Chemical Reaction
The most basic way to show how carbon dioxide is formed is:
Is Carbon Dioxide Harmful?
The air we breathe usually has a very small amount of carbon dioxide (about 0.036% to 0.041%).
Carbon dioxide is not usually considered poisonous in small amounts. However, in higher concentrations, it can be dangerous.
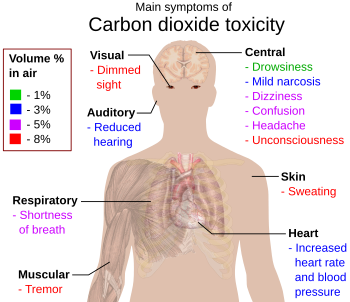
- If the air has about 1% carbon dioxide, some people might feel sleepy or their lungs might feel stuffy.
- At 7% to 10% concentration, carbon dioxide can cause suffocation, even if there's enough oxygen. Symptoms include dizziness, headache, problems with seeing and hearing, and even losing consciousness within minutes.
Because carbon dioxide is heavier than air, it can collect in low, sheltered places, especially where it seeps from the ground (like near volcanoes). This can be very dangerous for animals that get trapped there.
Important Safety Note: Poor air circulation in closed spaces can lead to too much carbon dioxide, which can make the air unhealthy to breathe.

Scientists have studied how long-term exposure to carbon dioxide affects people and animals, even at lower levels. For example, astronauts on the International Space Station experienced headaches, tiredness, and trouble thinking clearly when carbon dioxide levels were around 0.5%.
Carbon Dioxide in Your Body
Your body makes about 1 kilogram (2.3 pounds) of carbon dioxide every day! This carbon dioxide is carried through your blood and breathed out by your lungs.
How CO2 Travels in Your Blood
Carbon dioxide travels in your blood in a few ways:
- Most of it (about 70-80%) is changed into something called bicarbonate ions in your red blood cells.
- A small amount (5-10%) dissolves directly into your blood.
- Another small amount (5-10%) attaches to hemoglobin, which is the molecule in red blood cells that also carries oxygen.
When carbon dioxide attaches to hemoglobin, it actually makes it harder for oxygen to attach. This is helpful because it means oxygen is released where your body needs it most.
How CO2 Controls Your Breathing
Even though your body needs oxygen, it's actually the level of carbon dioxide in your blood that mostly tells you when to breathe.
Your brain's breathing centers try to keep the carbon dioxide level in your blood just right.
- If you breathe too slowly or shallowly, carbon dioxide builds up, and your body tells you to breathe more.
- If you breathe too fast (like during hyperventilation), you breathe out too much carbon dioxide, which can make you feel dizzy.
This is why if you hold your breath after breathing very fast, you can hold it longer. But this can be dangerous, especially if you're free diving, because you might pass out before you feel the need to breathe.
Images for kids
-
A fire extinguisher that uses carbon dioxide to put out fires.
See also
 In Spanish: Dióxido de carbono para niños
In Spanish: Dióxido de carbono para niños
 | Tommie Smith |
 | Simone Manuel |
 | Shani Davis |
 | Simone Biles |
 | Alice Coachman |