Iron facts for kids
- This article is about iron the metal. For the tool called iron, see ironing.
Iron (chemical symbol Fe) is a very common metal found on Earth. It's the most used metal in the world! Iron is element number 26 on the periodic table. Its symbol is Fe, which comes from the Latin word for iron, ferrum.
People use iron a lot because it is very strong and cheap to get. Iron is the main ingredient used to make steel, which is even stronger. Raw iron is attracted to magnets. It can also be used to make an electromagnet.
Contents
What is Iron Like?
Physical Features of Iron
Iron is a shiny, grey metal. It is magnetic. It is easy to dig up and shape, which makes it very useful. Pure iron is soft and can be stretched a lot. But when iron is mixed with a little carbon to make steel, it becomes much stronger and doesn't stretch as much.
How Iron Reacts (Chemical Properties)
Iron is a reactive metal. It reacts with most acids, like sulfuric acid. This reaction is sometimes used to clean other metals.
One important thing about iron is that it reacts with air and water to create rust. When rust flakes off, new iron is exposed, and it starts to rust too. Eventually, a whole piece of iron can rust away. Other metals, like aluminum, don't rust in the same way. To stop iron from rusting, it can be mixed with chromium and carbon to make stainless steel. Stainless steel usually doesn't rust.
Iron powder can also react with sulfur to make a hard black solid called iron(II) sulfide.
Iron Compounds
Iron can combine with other elements to form chemical compounds. When iron forms compounds, it often loses either two or three electrons.
- Compounds where iron loses two electrons are called ferrous compounds. Many of these are green or blue.
- Compounds where iron loses three electrons are called ferric compounds. Many of these are brown.
Ferric compounds are often found in rust. This is why if there's a small scratch in paint on an iron object, the whole thing can start to rust.
Common Iron Compounds
- Iron(II) sulfate: This is a blue-green chemical. It's made by reacting sulfuric acid with steel. It can be used to clean certain poisons.
- Iron(III) oxide: This is what we call rust. It's a red-brown substance that can dissolve in acid.
- Iron(III) chloride: This compound is poisonous and can cause damage. It dissolves in water to make a dark brown, acidic liquid.
Where We Find Iron
Scientists believe that the Earth's core is mostly made of iron metal. However, we almost never find pure iron metal on the Earth's surface. Sometimes, meteorites that fall to Earth contain iron.
Most of the time, iron is found in the ground as ore. The most common iron ore is called hematite. A lot of this ore was formed during a time called the Great Oxygenation Event, when oxygen first became common in Earth's atmosphere. Another type of iron ore is magnetite.
Iron is also important for our bodies! It's found in meat and in hemoglobin, which is a part of our red blood cells.
How We Make Iron
Iron is made in huge factories called ironworks. This process involves heating iron ore with carbon (which is usually coke, a type of coal). This happens inside very large containers called blast furnaces.
Here's how it works:
- The blast furnace is filled with iron ore, coke, and limestone.
- A very hot blast of air is blown in. This makes the coke burn.
- The extreme heat causes the carbon to react with the iron ore. The carbon takes the oxygen away from the iron oxides in the ore, creating carbon dioxide gas. This gas then leaves the mixture.
- There's also some sand mixed in with the iron ore. The limestone turns into calcium oxide when it gets very hot.
- The calcium oxide reacts with the sand to form a liquid called slag. This slag is drained away, leaving only the pure liquid iron.
- The liquid iron can then be shaped and hardened as it cools.
Today, almost all ironworks are part of steel mills, because most iron is turned into steel.
There are many ways to work with iron. You can make it harder by heating it and then quickly cooling it in cold water. You can make it softer by heating it and letting it cool down slowly. Iron can also be pressed into shapes or pulled into thin wires. It can even be rolled into flat sheets of metal.
What Is Iron Used For?
Iron as a Metal

Iron is used more than any other metal. It's strong and cheap. It's used to build many things like buildings, bridges, nails, screws, pipes, and towers.
Iron is not too reactive, which makes it easy and cheap to get from its ores. Once it's made into steel, it's very strong and is even used to make concrete stronger.
There are different kinds of iron:
- Cast iron is made by the process described above. It's hard but can break easily. It's used for things like manhole covers and parts of engines.
- Steel is the most common form of iron.
* Mild steel has a low amount of carbon. It's soft and can bend easily, but it doesn't crack much. It's used for nails and wires. * Carbon steel (with more carbon) is harder but can be more brittle. It's used for tools. * Stainless steel contains chromium, which helps it resist rust.
- Wrought iron is easy to shape. It's used to make things like fences and chains.
Very pure iron is soft and can rust easily.
Iron in Compounds
Iron compounds are used for different purposes.
- Iron(II) chloride and Iron(III) chloride are used to help clean water.
- Iron(II) sulfate is used in cement.
- Some iron compounds are even found in vitamins.
Iron in Our Food
Our bodies need iron to help oxygen get to our muscles. Iron is a key part of important molecules in our bodies, like hemoglobin in our blood.
Many cereals have extra iron added to them. This iron is in the form of tiny metal pieces. You can sometimes even see these tiny pieces if you put a strong magnet into a box of cereal – the magnet will attract them! Eating these small pieces of iron is not harmful to our bodies.
Iron from diet supplements is often in a chemical form, like Iron(II) sulfate, which is cheap and easy for the body to absorb. Our bodies are smart and won't take in more iron than they need. The iron in our red blood cells is also recycled when old cells break down.
Safety with Iron
Swallowing very large amounts of iron can be harmful and damage the body. If someone eats too many vitamins that contain iron, they can get sick. Doctors have special chemicals that can react with iron to help prevent poisoning.
Related pages
- Iron compounds
Images for kids
-
Ochre path in Roussillon.
-
Comparison of colors of solutions of ferrate (left) and permanganate (right)
-
Iron harpoon head from Greenland made from meteorite iron.
-
Coalbrookdale by Night, 1801. Blast furnaces light the iron making town of Coalbrookdale.
-
"Gold gab ich für Eisen" – "I gave gold for iron". German-American brooch from WWI.
-
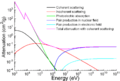
Photon mass attenuation coefficient for iron.
See also
 In Spanish: Hierro para niños
In Spanish: Hierro para niños
 | Aaron Henry |
 | T. R. M. Howard |
 | Jesse Jackson |