Permanganate facts for kids
Quick facts for kids Permanganate |
|
|---|---|
 |
|
 |
|
|
Permanganate
|
|
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
| Infobox references | |
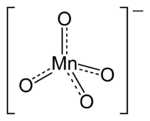
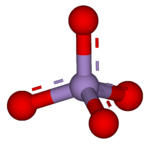
A permanganate is a special kind of ion. An ion is an atom or group of atoms that has an electric charge. This ion contains one manganese atom and four oxygen atoms. The manganese atom in permanganate has a very high oxidation state of +7. This means it has lost seven electrons.
Permanganates are known for being strong oxidizing agents. This means they can easily take electrons from other substances. This process is called oxidation. Permanganates are usually purple-black in color. They can also stain things easily. This happens because they are quickly changed into a brown-black substance called manganese dioxide. This change is a chemical reaction.
Contents
What are Permanganates Used For?
Permanganates are used in many different ways. They are often used in chemistry labs. They help to clean water and treat waste. They can also be used as disinfectants. This means they kill germs and bacteria.
Common Examples of Permanganates
- Potassium permanganate: This is the most common type of permanganate. It is often used in experiments and for water treatment.
Similar Chemical Ions
- Chromate: This is another ion that is similar to permanganate. It also contains a metal atom (chromium) and oxygen atoms.
- Manganese: This is the metal element found in permanganate.
- Manganese dioxide: This is the brown-black substance that forms when permanganate reacts.
Images for kids
See also
 In Spanish: Permanganato para niños
In Spanish: Permanganato para niños