Chromate facts for kids
Quick facts for kids Chromate |
|
|---|---|
 |
|
 |
|
|
Chromate and Dichromate
|
|
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
| Infobox references | |
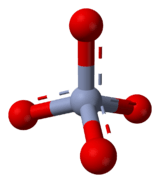
Chromate is a special kind of atom group called an ion. It has one chromium atom and four oxygen atoms. Its chemical formula is CrO4, and it has a charge of -2.
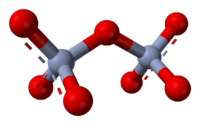
Dichromate is very similar. It has two chromium atoms and seven oxygen atoms. Its formula is Cr2O7, and it also has a charge of -2.
Chromate and dichromate are strong "oxidizers" when they are in acidic liquids. This means they can easily take electrons from other substances. But, they are weaker oxidizers in basic liquids. Chromates are usually bright yellow. Dichromates are often orange or red.
You can make these compounds by mixing chromium(III) oxide with a metal oxide in a basic environment. They can be broken down by reacting them with "reducing agents." These are substances that give electrons to other things. The oxygen in the air helps change the chromium atom from one form (+3) to another (+6).
Contents
What are Chromates and Dichromates?
Chromate and dichromate are chemical compounds that contain the element chromium. Chromium is a metal often used to make things shiny, like car parts. In these compounds, chromium is in a special state called its +6 oxidation state. This means it has lost six electrons.
These compounds are important in chemistry because of how they react. They are known for their bright colors. This makes them useful in some industrial processes.
How are they made and used?
Chromates and dichromates are often made in factories. They are created by combining chromium(III) oxide with other metal oxides. This process usually happens in a basic, or alkaline, environment.
These chemicals have been used in different ways. For example, they were once used as pigments to make paints and dyes. They were also used in some types of leather tanning. However, because of safety concerns, their use has become more limited.
Safety and Health
It's important to know that chromates and dichromates can be harmful. When these compounds are in powder form, their dust can be dangerous. Breathing in this dust can cause cancer. This is why they are called carcinogenic.
To make them safer, chemists can change chromate into a less harmful form. This is done by a process called reduction. For example, you can use something like iron(II) sulfate to reduce chromate. This changes the chromium to a different, safer state.
When working with these chemicals, it's very important to follow strict safety rules. This includes wearing protective gear.
Examples of these compounds
Here are some common examples of chromates and dichromates:
- Chromates
- Dichromates
Related pages

