Chromium facts for kids
Chromium is a special kind of building block called a chemical element. You can find it on the periodic table, which is like a big list of all known elements. Its symbol there is Cr. Every atom of chromium always has 24 tiny particles called protons. This means its atomic number is 24. Most chromium atoms also have about 28 neutrons, but some can have a few more or less. These different versions are called isotopes. When chromium is a metal, it has 24 electrons. But when it forms ions, it has fewer electrons.
Contents
What is Chromium Like?
Chromium is a very shiny metal. It likes to react with other things, especially air. But when it reacts with air, it quickly forms a very thin, invisible layer of chromium(III) oxide. This special coating acts like a shield, stopping the metal from rusting or reacting any further.
Chromium Compounds: Colorful Chemicals
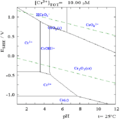
Chromium can exist in different forms, called oxidation states. The most common forms are +2, +3, and +6. When chromium combines with other elements, it creates chemical compounds that are often very colorful!
Here are a few examples of chromium compounds:
- Chromium(II) oxide: This compound is black. It's not very common.
- Chromium(III) oxide: This one is a dark green color.
- Chromium(III) chloride: This can be green if it has water in it, or purple if it's completely dry.
- Chromium(VI) oxide: Also known as chromium trioxide, this compound is red. It's a strong chemical and can be harmful. When it dissolves in water, it forms Chromic acid.
- Chromyl chloride: This is a red liquid.
Trivalent Chromium: Good for You!
There are two main types of chromium that are important to know about. One type is called trivalent chromium (written as Cr3+). This form of chromium is actually needed by our bodies and the bodies of other animals. If we don't get enough of it, some parts of our body might not work as well as they should.
We usually get the chromium we need from the food we eat. Sometimes, it's also added to vitamins to make sure people get enough. While it's good for us, getting too much of this type of chromium can still be harmful.
Hexavalent Chromium: The Harmful Kind
The other type of chromium is called hexavalent chromium (written as Cr6+). This form can be very harmful and can even cause serious sickness. Most people don't come into contact with it very often.
Hexavalent chromium is often found in places where chromium metal is made. Because it's dangerous, these places need to be carefully cleaned up when they close down. Luckily, hexavalent chromium can be changed into the safer trivalent chromium (Cr3+) by reacting it with certain chemicals.
- Chromates
A chromate is a chemical that contains the chromate ion (CrO42-). These compounds are usually yellow.
- Dichromates
A dichromate is a chemical that contains the dichromate ion (Cr2O72-). These compounds are typically red or orange.
- Ammonium dichromate
- Potassium dichromate
- Sodium dichromate: This is used when making chromium.
Where is Chromium Found and How is it Made?
Chromium is found in a mineral called chromite. Chromite is a natural mix of iron(II) oxide and chromium(III) oxide. Its chemical formula is FeCr2O4.
To get chromium metal, chromite is first heated with sodium carbonate. This creates sodium chromate, along with iron(III) oxide and carbon dioxide. The sodium chromate is then treated with sulfuric acid to make sodium dichromate. Next, the sodium dichromate is changed into chromium(III) oxide using carbon. Finally, the chromium(III) oxide is reacted with aluminum to produce pure chromium metal.
The chromates and dichromates used in this process are what make chromium production areas potentially harmful.
What is Chromium Used For?
Chromium is used in many different metal products. One of its most common uses is in stainless steel, which is used for things like kitchen sinks and cutlery because it doesn't rust.
It's also used in something called "chrome plating." This is a process where a thin layer of chromium is put on other metals to make them shiny and stop them from rusting. You might see chrome plating on car parts or motorcycle parts.
Some chromium compounds were once used as colorful paints, but because they can be harmful, people don't use them for that purpose much anymore.
Is Chromium Safe?
Pure chromium metal itself is not harmful. However, as we learned, hexavalent chromium is very harmful and can cause serious health problems. Trivalent chromium, which our bodies need, can also be mildly harmful if you get too much of it. It's important to handle chromium compounds carefully, especially the hexavalent form.
Related pages
- Chromium compounds
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrogen & alkali metals |
Alkaline earth metals | Triels | Tetrels | Pnictogens | Chalcogens | Halogens | Noble gases |
||||||||||||
| Period |
|||||||||||||||||||
| 2 | |||||||||||||||||||
| 3 | |||||||||||||||||||
| 4 | |||||||||||||||||||
| 5 | |||||||||||||||||||
| 6 | |||||||||||||||||||
| 7 | |||||||||||||||||||
Primordial From decay Synthetic Border shows natural occurrence of the element
- Ca: 40.078 — Abridged value (uncertainty omitted here)
- Po: [209] — mass number of the most stable isotope
Images for kids
-
Sodium chromate (Na2CrO4)
See also
 In Spanish: Cromo para niños
In Spanish: Cromo para niños