Arsenic facts for kids
Arsenic is a special chemical element with the symbol As and atomic number 33. You can find it on the periodic table in Group 15, which is called the pnictogen group. Its atomic mass is about 74.92.
Contents
What is Arsenic Like?
Different Forms of Arsenic
Arsenic can show up in three different forms, called allotropes.
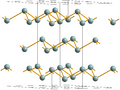
- Gray arsenic is the most common form. It's a bit shiny and looks like a metalloid, which means it has properties of both metals and nonmetals. It's also brittle, meaning it can break easily. This form can conduct some electricity, making it a semiconductor.
- Yellow arsenic is very rare and doesn't last long. It's the most toxic (poisonous) type. It's soft and waxy, a bit like white phosphorus. If light hits it, it quickly changes into gray arsenic.
- Black arsenic is brittle, black, and shiny. It doesn't conduct electricity well. It's similar to red phosphorus.
Since gray arsenic is found most often, when people say "arsenic," they usually mean this form.
Arsenic's Atoms
Arsenic has one stable isotope called 75As. This means it's not radioactive. However, there are also about 33 radioactive isotopes of arsenic. The one that lasts the longest is 73As, which has a half-life of about 80 days.
How Arsenic Reacts
Arsenic doesn't react much with other chemicals, similar to copper. When it burns in the air, it creates a substance called arsenic trioxide, which smells like garlic.
An interesting thing about arsenic and some of its compounds is that they don't have a liquid state. Instead, when heated, they go straight from a solid to a gas. This process is called subliming.
Arsenic can react with halogens (like fluorine) to make different compounds. For example, it reacts with fluorine to make arsenic pentafluoride. It doesn't dissolve in hydrochloric acid but will dissolve in strong nitric acid or sulfuric acid.
Arsenic's Compounds
Arsenic forms many chemical compounds. These compounds can be grouped by how arsenic shares or takes electrons, which chemists call oxidation states. The main states are -3, +3, and +5.
- Compounds in the -3 state are often found in the ground as arsenides. An example is Arsine, which is a very toxic gas that smells like garlic. Gallium arsenide is another important -3 compound used in things like LEDs.
- Compounds in the +3 state are the most common. Arsenic trioxide is a white solid in this group. When it dissolves in water, it forms arsenious acid.
- Compounds in the +5 state are also common. Arsenic pentafluoride is a colorless gas in this group. Arsenic pentoxide is another white solid that dissolves in water to make arsenic acid. This acid can then form arsenate salts.
Some examples of arsenic compounds include:
- Arsenides: Gallium arsenide (used in LEDs), Arsine (a toxic gas).
- Arsenites: These are salts of arsenious acid. Paris green (copper acetoarsenite) is a green solid that was once used as a pigment.
- Arsenates: These are salts of arsenic acid. Lead arsenate was used as an insecticide.
Where is Arsenic Found?
Arsenic is sometimes found as a pure element in the ground, but it's usually part of minerals. Many arsenic minerals contain a metal and sulfur along with arsenic. For example, arsenopyrite is a mineral made of iron, arsenic, and sulfur. Other minerals, like erythrite, contain a metal and arsenic, such as cobalt arsenide.
Some simple arsenic minerals, like Realgar and orpiment (which are both arsenic sulfides), are important ores from which arsenic is extracted.
You can also find small amounts of a less toxic type of arsenic in fish and mushrooms. Some scientists believe that humans might need tiny amounts of arsenic to stay healthy. There are even some bacteria that can use arsenic instead of phosphorus for certain things!
How is Arsenic Made?
Most arsenic is not mined directly. Instead, it's often collected from the waste products left over after other metals are processed. Only China still actively mines arsenic.
One way to get arsenic is by heating arsenopyrite. This process creates arsenic trioxide, which turns into a gas and then condenses back into a solid. This solid can then be reduced (changed) using carbon to make pure arsenic. Another method is to heat arsenopyrite without air, which directly produces gray arsenic.
A Look Back: Arsenic's History
People have known about arsenic since ancient times. It was mixed with bronze to make the bronze stronger. Some people even used arsenic in makeup, even though it was very toxic!
Arsenic became famous as a poison. Because it was hard to detect, people sometimes used it to harm royalty. This led to it being called the "Poison of Kings" and the "King of Poisons."
What is Arsenic Used For?
In the past, arsenic compounds were used to protect wood from rotting, acting as a preservative. However, once people realized how toxic arsenic was, they stopped using it for wood. Some animal feed also contained arsenic to prevent diseases. Lead arsenate was used as an insecticide in orchards, but it was harmful to people who applied it.
Arsenic was also used in medicines during the 1700s, 1800s, and 1900s. For example, arsenic trioxide was used to treat some types of cancer. Very small amounts of arsenic compounds could even act as stimulants. Some arsenic compounds were also used in poison gases.
Today, arsenic as an element is used in alloys. For example, a small amount of arsenic is added to the lead in lead-acid batteries to make them stronger. It's also used in some semiconductors, which are important parts of electronic devices.
In the past, copper arsenate was used to color sweets, and Paris green was a green pigment that made many people sick. Some bullets also contain arsenic. Arsenic was once used in optical glass but was removed due to its toxicity.
Is Arsenic Safe?
Arsenic and its compounds are very toxic (poisonous) and can cause cancer. Water near arsenic mines can often become polluted with arsenic. Wood that was preserved with arsenic compounds can also release arsenic into the soil. Even emissions from coke ovens can contain arsenic.
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrogen & alkali metals |
Alkaline earth metals | Triels | Tetrels | Pnictogens | Chalcogens | Halogens | Noble gases |
||||||||||||
| Period |
|||||||||||||||||||
| 2 | |||||||||||||||||||
| 3 | |||||||||||||||||||
| 4 | |||||||||||||||||||
| 5 | |||||||||||||||||||
| 6 | |||||||||||||||||||
| 7 | |||||||||||||||||||
Primordial From decay Synthetic Border shows natural occurrence of the element
- Ca: 40.078 — Abridged value (uncertainty omitted here)
- Po: [209] — mass number of the most stable isotope
Images for kids
-
Crystal structure common to Sb, AsSb and gray As
-
The arsenic labyrinth, part of Botallack Mine, Cornwall
-
Satirical cartoon by Honoré Daumier of a chemist giving a public demonstration of arsenic, 1841
See also
 In Spanish: Arsénico para niños
In Spanish: Arsénico para niños
 | Selma Burke |
 | Pauline Powell Burns |
 | Frederick J. Brown |
 | Robert Blackburn |