Neodymium facts for kids
 |
|||||||||||||||
| Neodymium | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˌniːoʊˈdɪmiəm/ |
||||||||||||||
| Appearance | silvery white | ||||||||||||||
| Standard atomic weight Ar, std(Nd) | 144.242(3) | ||||||||||||||
| Neodymium in the periodic table | |||||||||||||||
|
|||||||||||||||
| Atomic number (Z) | 60 | ||||||||||||||
| Group | n/a | ||||||||||||||
| Period | period 6 | ||||||||||||||
| Block | f | ||||||||||||||
| Electron configuration | [Xe] 4f4 6s2 | ||||||||||||||
| Electrons per shell | 2, 8, 18, 22, 8, 2 | ||||||||||||||
| Physical properties | |||||||||||||||
| Phase at STP | solid | ||||||||||||||
| Melting point | 1295 K (1022 °C, 1872 °F) | ||||||||||||||
| Boiling point | 3347 K (3074 °C, 5565 °F) | ||||||||||||||
| Density when liquid (at m.p.) | 6.89 g/cm3 | ||||||||||||||
| Heat of fusion | 7.14 kJ/mol | ||||||||||||||
| Heat of vaporization | 289 kJ/mol | ||||||||||||||
| Molar heat capacity | 27.45 J/(mol·K) | ||||||||||||||
Vapor pressure
|
|||||||||||||||
| Atomic properties | |||||||||||||||
| Oxidation states | +2, +3, +4 (a mildly basic oxide) | ||||||||||||||
| Electronegativity | Pauling scale: 1.14 | ||||||||||||||
| Ionization energies |
|
||||||||||||||
| Atomic radius | empirical: 181 pm | ||||||||||||||
| Covalent radius | 201±6 pm | ||||||||||||||
| Spectral lines of neodymium | |||||||||||||||
| Other properties | |||||||||||||||
| Natural occurrence | primordial | ||||||||||||||
| Crystal structure | double hexagonal close-packed (dhcp) | ||||||||||||||
| Speed of sound thin rod | 2330 m/s (at 20 °C) | ||||||||||||||
| Thermal conductivity | 16.5 W/(m⋅K) | ||||||||||||||
| Electrical resistivity | poly: 643 nΩ⋅m | ||||||||||||||
| Magnetic ordering | paramagnetic, antiferromagnetic below 20 K | ||||||||||||||
| Molar magnetic susceptibility | +5628.0×10−6 cm3/mol (287.7 K) | ||||||||||||||
| Young's modulus | 41.4 GPa | ||||||||||||||
| Shear modulus | 16.3 GPa | ||||||||||||||
| Bulk modulus | 31.8 GPa | ||||||||||||||
| Poisson ratio | 0.281 | ||||||||||||||
| Vickers hardness | 345–745 MPa | ||||||||||||||
| Brinell hardness | 265–700 MPa | ||||||||||||||
| CAS Number | 7440-00-8 | ||||||||||||||
| History | |||||||||||||||
| Discovery | Carl Gustaf Mosander (1841) | ||||||||||||||
| First isolation | Carl Auer von Welsbach (1885) | ||||||||||||||
| Named by | Carl Auer von Welsbach (1885) | ||||||||||||||
|
|||||||||||||||
Neodymium is a chemical element with the symbol Nd and atomic number 60. It's part of the lanthanide series and is known as a rare-earth metal. Neodymium is a hard, shiny, silvery metal. It can be shaped easily. However, it quickly gets dull and rusty when exposed to air and moisture.
When neodymium reacts with oxygen, it forms colorful compounds. These can be pink, purple/blue, or yellow. Neodymium has one of the most complex light patterns (spectra) of all elements. An Austrian chemist named Carl Auer von Welsbach discovered it in 1885. He also discovered praseodymium.
You can find neodymium in minerals like monazite and bastnäsite. It's never found naturally as a pure metal. It's usually mixed with other lanthanides. Neodymium is quite common, similar to cobalt, nickel, or copper. It's found all over the Earth's crust. Most of the world's neodymium comes from mines in China.
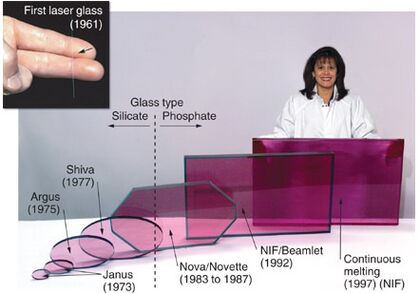
Neodymium compounds were first used in 1927 to color glass. They are still popular for this today. Neodymium gives glass a reddish-purple color. This color can change depending on the type of light. This happens because neodymium absorbs certain colors of light. Glass with neodymium is used in powerful lasers. These lasers are used in very high-power jobs, like in inertial confinement fusion. Neodymium is also used in other lasers, such as the Nd:YAG laser.
Neodymium alloys (mixtures of metals) make very strong neodymium magnets. These are super powerful permanent magnets. You can find these magnets in many products. Examples include microphones, loudspeakers, headphones, and computer hard drives. They are used where strong magnets are needed in a small space. Bigger neodymium magnets are used in electric motors. These motors are found in hybrid cars and wind turbine electric generators.
Contents
- What are the physical properties of Neodymium?
- What are the chemical properties of Neodymium?
- What are Neodymium isotopes?
- How was Neodymium discovered?
- Where is Neodymium found and how is it made?
- What are the uses of Neodymium?
- What are the biological effects and safety precautions for Neodymium?
- See also
What are the physical properties of Neodymium?
Metallic neodymium looks bright and silvery. Neodymium exists in two main forms. It changes from one form to another at about 863 °C. Neodymium is paramagnetic at room temperature. This means it is weakly attracted to magnets. When it gets very cold (below -253 °C), it becomes an antiferromagnet. This means its tiny magnetic parts line up in opposite directions.
How are Neodymium's electrons arranged?
Neodymium is the fourth element in the lanthanide series. In the periodic table, it sits between praseodymium and promethium. Its 60 electrons are arranged in a specific way. Six of these electrons are "valence" electrons. These are the ones involved in chemical reactions. Like most lanthanides, neodymium usually uses three valence electrons. However, it can sometimes lose a fourth electron.
What are the chemical properties of Neodymium?
Neodymium melts at 1024 °C and boils at 3074 °C. Like other lanthanides, it usually has a +3 oxidation state. This means it tends to lose three electrons in reactions. But it can also form +2 or +4 states. Neodymium metal quickly rusts in normal air. It forms an oxide layer that can peel off. This exposes more metal to rust. A piece of neodymium the size of your finger can completely rust away in about a year.
Neodymium reacts with water to form neodymium(III) hydroxide:
- 2Nd (s) + 6H
2O (l) → 2Nd(OH)
3 (aq) + 3H
2 (g)
It also reacts strongly with halogens like fluorine, chlorine, bromine, and iodine:
- 2Nd (s) + 3F
2 (g) → 2NdF
3 (s) (a violet substance) - 2Nd (s) + 3Cl
2 (g) → 2NdCl
3 (s) (a mauve substance) - 2Nd (s) + 3Br
2 (g) → 2NdBr
3 (s) (a violet substance) - 2Nd (s) + 3I
2 (g) → 2NdI
3 (s) (a green substance)
Neodymium dissolves easily in weak sulfuric acid. This forms a lilac-colored solution.
What are some Neodymium compounds?
Some important neodymium compounds include:
- Halides: NdF3, NdCl2, NdCl3, NdBr3, NdI2, NdI3
- Oxides: Nd
2O
3 - Hydroxide: Nd(OH)
3 - Carbonate: Nd2(CO3)3
- Sulfate: Nd
2(SO
4)
3 - Acetate: Nd(CH3COO)3
- neodymium magnets (Nd2Fe14B)
Some neodymium compounds change color under different types of light.
-
Neodymium compounds in fluorescent tube light—from left to right, the sulfate, nitrate, and chloride
-
Neodymium compounds in compact fluorescent lamp light
What are Neodymium isotopes?
Naturally, neodymium has five stable forms called isotopes. These are 142Nd, 143Nd, 145Nd, 146Nd, and 148Nd. 142Nd is the most common. It also has two radioisotopes that last a very long time: 144Nd and 150Nd. Scientists have found 33 different radioisotopes of neodymium in total. Most of these are very short-lived.
Neodymium isotopes are used in science. For example, 142Nd helps make other short-lived isotopes. 146Nd is used to make 147Pm, which can be a source of radioactive power. The decay of 147Sm to 143Nd helps scientists figure out the age of rocks and meteorites. This is called samarium–neodymium dating.
How was Neodymium discovered?
In 1751, a Swedish scientist found a heavy mineral. It was later named cerite. In 1803, Jöns Jacob Berzelius and Wilhelm Hisinger found a new oxide from this mineral. They called it ceria. Later, between 1839 and 1843, another Swedish chemist, Carl Gustaf Mosander, showed that ceria was a mixture. He separated two other oxides, which he named lanthana and didymia. The metals from these oxides were called lanthanum and didymium.
In 1885, Carl Auer von Welsbach officially discovered neodymium. He separated didymium into two new elements. He named them neodymium and praseodymium. The name neodymium comes from Greek words meaning "new twin." Pure neodymium was finally separated in 1925.
For a long time, neodymium was purified using a method called double nitrate crystallization. After World War II, a new method called ion exchange was developed. This allowed for much purer neodymium. Today, most neodymium is taken from bastnäsite and purified using a process called solvent extraction.
Where is Neodymium found and how is it made?
Where is Neodymium found?
Neodymium is rarely found as a pure element in nature. Instead, it's in ores like monazite and bastnäsite. These minerals contain small amounts of all rare-earth elements. The main places where neodymium is mined are China, the United States, Brazil, India, Sri Lanka, and Australia.
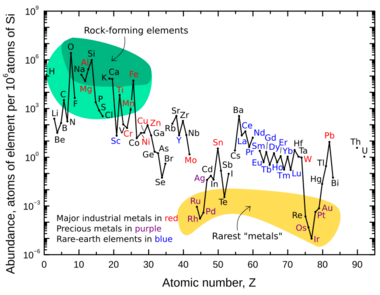
Neodymium is a lithophile element. This means it's usually found combined with oxygen. Even though it's a "rare-earth metal," neodymium is not actually rare. It's quite common in the Earth's crust. It's about as common as lanthanum.
| Atomic number |
Element | Relative amount |
|---|---|---|
| 42 | Molybdenum | 2.771 |
| 47 | Silver | 0.590 |
| 50 | Tin | 4.699 |
| 58 | Cerium | 1.205 |
| 59 | Praseodymium | 0.205 |
| 60 | Neodymium | 1 |
| 74 | Tungsten | 0.054 |
| 90 | Thorium | 0.054 |
| 92 | Uranium | 0.022 |
How is Neodymium produced?
About 7,000 tons of neodymium were produced worldwide in 2004. Most of it comes from China. The Chinese government used to control the supply of neodymium. This caused its prices to change a lot. This made companies look for ways to make magnets with less rare-earth metals. However, they still need neodymium for strong magnets.
The US Geological Survey says Greenland has the largest untouched rare-earth deposits. These include a lot of neodymium. But mining there can cause problems. It can release radioactive substances like thorium. This can affect local communities.
Neodymium makes up 10–18% of the rare-earth content in minerals like bastnäsite and monazite. Neodymium compounds are the most colorful of the trivalent lanthanides. They often give a pink color to rare-earth minerals. These minerals can also change color under different lights. For example, they might look green under certain UV light.
The demand for rare-earth elements like neodymium is growing fast. This is due to more people and more industries. There's also a big need for energy-saving technologies. These include batteries, efficient motors, and renewable energy. NdFeB magnets are key for making high-efficiency motors. They are used in hybrid electric vehicles, electric vehicles, wind turbines, and many electronic devices. These magnets are important for saving energy. Their demand is expected to increase a lot in the future.
What are the uses of Neodymium?
Magnets

Neodymium magnets are the strongest permanent magnets known. A small neodymium magnet can lift a thousand times its own weight. They can snap together with enough force to cause injuries. These magnets are cheaper, lighter, and stronger than other types of magnets. However, they lose their magnetism at lower temperatures and can rust.
Neodymium magnets are used in many products. These include microphones, loudspeakers, headphones, and computer hard disks. They are chosen when a strong magnetic field is needed in a small space. They are also used in the electric motors of hybrid and electric cars. Some wind turbines also use neodymium in their generators. For example, a Toyota Prius car needs about 1 kilogram (2.2 pounds) of neodymium.
Glass

Neodymium glass is made by adding neodymium oxide to melted glass. In daylight, neodymium glass looks lavender. But under fluorescent lighting, it appears pale blue. Neodymium can color glass in shades from violet to wine-red or warm gray.
The first use of purified neodymium for glass coloring was in 1927. This "Alexandrite" glass is still famous today. Many glass companies copied this in the 1930s. The color of neodymium glass changes with different lighting. It looks reddish-purple in daylight or yellow light. It looks blue under white fluorescent light. It can even look greenish under special lighting.
Neodymium glass has very sharp light absorption bands. This makes it useful in astronomical work. It helps astronomers calibrate spectral lines. It's also used in special filters to reduce light pollution. These filters block light from sodium and fluorescent lamps. This helps astronomers see other colors better. Neodymium also helps remove green color caused by iron in glass.
Neodymium is part of "didymium" glass. This glass is used for welder's and glass-blower's goggles. It blocks the strong yellow light from sodium. This protects their eyes. Neodymium glass is also used in light bulbs. These bulbs filter out yellow light, making the light appear whiter and more like sunlight. Neodymium salts are also used to color enamels.
Lasers
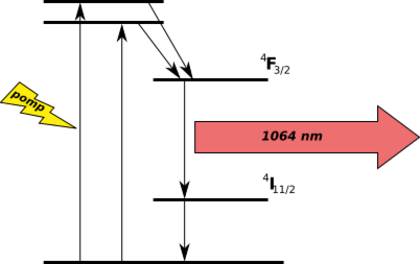
Some clear materials with neodymium ions are used in lasers. These lasers produce infrared light. Examples include Nd:YAG and Nd:glass lasers. Neodymium-doped crystals are used in commercial green laser pointers.
The neodymium ion (Nd3+) was the first rare-earth element used in lasers. The Nd:CaWO4 laser was made in 1961. It was the third laser ever made. Over time, neodymium lasers became very common. They are used for many different purposes.
Very powerful neodymium glass lasers are used in inertial confinement fusion. These lasers are extremely strong. They can create plasmas as hot as 1 million K. This helps scientists study how density, temperature, and pressure work inside warheads.
Other uses of Neodymium
- Neodymium has a very large specific heat capacity at very cold temperatures. This makes it useful in cryocoolers.
- Neodymium acetate can be used in electron microscopy. It helps make images clearer.
- Some studies suggest that Nd3+ can help plants grow. Rare-earth elements are often used as fertilizer in China.
- Samarium–neodymium dating helps determine the age of rocks and meteorites.
- Neodymium isotopes in ocean sediments help scientists understand past ocean currents.
What are the biological effects and safety precautions for Neodymium?
| Neodymium | |
|---|---|
| Hazards | |
| NFPA 704 |
|
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Some bacteria living in volcanic mudpots need early lanthanides, including neodymium, to survive. Other than that, neodymium is not known to have a role in living things.
Neodymium metal dust can catch fire and cause an explosion. Neodymium compounds are not very toxic. However, their effects haven't been fully studied. Soluble neodymium salts are considered more toxic than insoluble ones. Neodymium dust and salts can irritate the eyes and nose. They can also irritate the skin. Breathing the dust can harm the lungs. Long-term exposure can damage the liver. Neodymium can also affect blood clotting.
Neodymium magnets are very strong. If not handled carefully, they can snap together quickly and forcefully. This can cause injuries. There's a story of someone losing a fingertip when two magnets snapped together from far away. Another danger is if someone swallows more than one magnet. They can pinch soft tissues in the body. This has led to many emergency room visits. Some toys made of small neodymium magnets have even been recalled because of this risk.
See also
 In Spanish: Neodimio para niños
In Spanish: Neodimio para niños
- Neodymium compounds
- Lanthanides
- Period 6 elements
- Rare earth metals
 | Janet Taylor Pickett |
 | Synthia Saint James |
 | Howardena Pindell |
 | Faith Ringgold |