Selenium facts for kids
Selenium is a special kind of building block called a chemical element. You can find it on the periodic table with the symbol Se. Its atomic number is 34, which means it has 34 tiny particles called protons in its center. It also has 34 electrons, which are even smaller particles that orbit the center. The average weight of a selenium atom is about 78.96.
Contents
Properties of Selenium
Physical Properties
Selenium atoms can arrange themselves in different ways. These different forms are called allotropes. The most common and stable form is a dense, gray material that looks a bit like a metal. Scientists call it a semimetal because it acts partly like a metal and partly like a nonmetal.
This gray form of selenium is special because it lets electricity flow through it much better when light shines on it. This unique feature makes it useful in devices like photocells, which are used to detect light.
Selenium also has other forms that don't conduct electricity well. One is a black, shiny substance that looks like glass. There are also several red forms that are made of tiny crystals. In these red forms, eight selenium atoms link together to form a ring-shaped molecule. These rings then stack up to create the solid red selenium. This structure is similar to how sulfur atoms are arranged.
Chemical Properties

Selenium is not a very reactive element. This means it doesn't easily combine with many other substances. It usually doesn't dissolve in most acids, except for a strong one called nitric acid. It can also dissolve in substances like sodium hydroxide, which are called alkalis.
When selenium powder is exposed to air, it reacts to form selenium(IV) oxide. Most selenium compounds are made from this selenium(IV) oxide because selenium itself doesn't react with many things.
Selenium can exist in different "states" when it forms compounds. These are called oxidation states. The main ones are -2, +2, +4, and +6.
- The -2 state is found in compounds called selenides. These are strong reducing agents, which means they are good at giving away electrons in chemical reactions. A common selenide is hydrogen selenide. Selenium also forms selenides when it reacts with active metals like sodium selenide or aluminium selenide.
- The +1 state is less common but can be found in compounds like selenium(I) chloride. These are generally very unreactive.
- The +4 state is found in compounds called selenites. These include selenous acid. Selenites are moderate oxidizing agents, meaning they can take electrons from other substances. They are made by dissolving selenium dioxide in water to form selenous acid, which then reacts with bases.
- The +6 state is found in compounds called selenates. These include selenic acid. Selenates are very powerful oxidizing agents. In fact, selenic acid is so strong it can even dissolve gold! They are made by reacting hydrogen peroxide with selenium(IV) oxide to get selenium(VI) oxide, which then dissolves in water to form selenic acid. Selenates are generally more reactive than similar compounds called sulfates.
Most selenium compounds don't have a color. They are also not very common to find.
Types of Selenium Compounds
- Selenides (from the -2 state): These are strong reducing agents.
- Selenide, the ion
- Hydrogen selenide
- +4 Compounds: These can be found as both negative and positive ions.
- Selenites (from the +4 state): These are weak oxidizing agents.
- Selenite, the ion
- Selenous acid
- +6 Compounds: These can also be found as both negative and positive ions.
- Selenates (from the +6 state): These are powerful oxidizing agents.
- Selenate, the ion
- Selenic acid
Where Selenium is Found
Selenium is very rarely found as a pure element in nature. Most of the selenium in soil is in tiny amounts and can be easily washed away. It's also found in very small amounts in the human body.
Selenium is most commonly found mixed in with sulfide ores, like pyrite, which are used to get metals. When these ores are processed, selenium is often collected as a byproduct. Sometimes, plants can absorb and concentrate selenium from the soil. Also, when copper is mined, selenium can sometimes wash into rivers, and too much selenium can be harmful to the river's ecosystem. Some types of coal also contain selenium.
How Selenium is Made
Selenium is usually made as a byproduct when companies refine copper or other sulfide ores. The process involves changing selenide ores into selenium dioxide. This selenium dioxide is then dissolved in acidic water to create selenous acid. Finally, sulfur dioxide is added to the selenous acid, which turns it into pure selenium. The selenium made this way is usually the red form. To get the black form, the red selenium is heated and melted.
Uses of Selenium
In Materials
Selenium is used in photocells because its electrical conductivity changes when light hits it. This is the gray, metallic form of selenium. Selenium can also act as a catalyst, which helps speed up chemical reactions.
One of the biggest uses for selenium is to give glass a red color. It can also be used in special types of brass instead of lead. Some older electronic parts called rectifiers used selenium, though most now use silicon. Selenium is also used to improve the look of photographs. You might even find selenium sulfide in some shampoos, as it helps remove dandruff.
In the Human Body
Selenium is a trace element for humans. This means our bodies need very small amounts of it to stay healthy, usually only about 50 to 200 micrograms each day.
However, too much selenium can be harmful. If someone takes more than 400 micrograms, it can become toxic. There was a time when people got selenium poisoning from coal that had a lot of selenium in it. While selenium has some helpful effects, it's important to consume only a very small amount.
Safety of Selenium
Selenium can be toxic if a person consumes large amounts. Some selenium compounds are also very harmful to living things in water. Consuming around 5 milligrams of selenium per day for a period of time can be dangerous for a human.
Related pages
- Selenium compounds
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrogen & alkali metals |
Alkaline earth metals | Triels | Tetrels | Pnictogens | Chalcogens | Halogens | Noble gases |
||||||||||||
| Period |
|||||||||||||||||||
| 2 | |||||||||||||||||||
| 3 | |||||||||||||||||||
| 4 | |||||||||||||||||||
| 5 | |||||||||||||||||||
| 6 | |||||||||||||||||||
| 7 | |||||||||||||||||||
Primordial From decay Synthetic Border shows natural occurrence of the element
- Ca: 40.078 — Abridged value (uncertainty omitted here)
- Po: [209] — mass number of the most stable isotope
Images for kids
-
Native selenium in sandstone, from a uranium mine near Grants, New Mexico
-
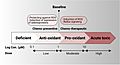
Selenium at nutritional levels or low concentrations is required for cell homeostasis, playing a role as an anti-oxidant through selenoproteins, thus, act chemo-preventive against cancer. In contrast, supra-nutritional levels or higher concentrations act as pro-oxidant in tumour cells, thus can be exploited as chemo-therapeutic against cancer.
-
Relationship between survival of juvenile salmon and concentration of selenium in their tissues after 90 days (Chinook salmon) or 45 days (Atlantic salmon) exposure to dietary selenium. The 10% lethality level (LC10=1.84 µg/g) was derived by applying the biphasic model of Brain and Cousens to only the Chinook salmon data. The Chinook salmon data comprise two series of dietary treatments, combined here because the effects on survival are indistinguishable.
See also
 In Spanish: Selenio para niños
In Spanish: Selenio para niños