Hydrogen peroxide facts for kids
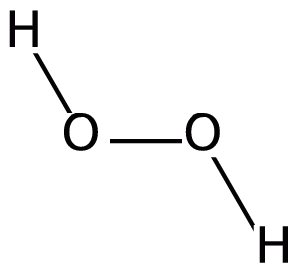
Hydrogen peroxide is a special chemical compound. Its chemical formula is H2O2. This means each molecule has two hydrogen atoms and two oxygen atoms. It's often used around the house as a cleaner or to bleach hair.
You might have seen it used to clean small cuts or scrapes. When it's used for wounds, it's usually a very weak mix, about 3% hydrogen peroxide and 97% water. Over time, hydrogen peroxide slowly breaks down into oxygen gas and water. This is why you might see bubbles when it's used on a cut!
Contents
What is Hydrogen Peroxide Used For?
Hydrogen peroxide has many uses, from cleaning to helping in science.
Cleaning and Disinfecting
Hydrogen peroxide is a great cleaner. It can kill germs and bacteria, which is why it's used as a disinfectant. It's often found in household cleaning products.
Hair Bleaching
Many people use hydrogen peroxide to lighten their hair. It works by removing the natural color from hair strands. This is why it's a common ingredient in hair dyes and bleaches.
Treating Wounds
A weak solution of hydrogen peroxide can be used to clean minor cuts and scrapes. It helps to remove dirt and dead tissue, which can prevent infection.
How Hydrogen Peroxide Works in Chemistry
Chemists use hydrogen peroxide in many chemical reactions. It can act in two main ways: as an "oxidizing agent" or a "reducing agent."
Oxidizing Agent
When hydrogen peroxide acts as an oxidizing agent, it takes electrons from other chemicals. Think of it like a magnet pulling something away. This process can change how other chemicals behave. For example, it can help turn one type of iron into another.
Reducing Agent
Hydrogen peroxide can also be a reducing agent. This means it gives electrons to other chemicals. When it does this, it often creates oxygen gas. Scientists sometimes use this method to make oxygen in a laboratory.
Safety Tips for Hydrogen Peroxide
You can buy hydrogen peroxide in stores, usually mixed with a lot of water. However, it can be very dangerous if it's too strong.
Handling Strong Solutions
Stronger mixes of hydrogen peroxide can be harmful. They can cause burns if they touch your skin. Always be careful and have an adult help you if you are using anything stronger than the common 3% solution.
Flammability and Storage
Hydrogen peroxide is not easily flammable on its own, but it can make other things burn more easily. It's important to store it safely, away from heat and light, because it breaks down over time.
Images for kids
-
The Australian bombardier beetle uses hydrogen peroxide as a defense.
-
Contact lenses soaking in a 3% hydrogen peroxide-based solution. The case includes a special disc that neutralizes the hydrogen peroxide over time.
-
Chemiluminescence of cyalume, as found in a glow stick. Hydrogen peroxide is often used to make glow sticks light up.
See also
 In Spanish: Peróxido de hidrógeno para niños
In Spanish: Peróxido de hidrógeno para niños
 | Stephanie Wilson |
 | Charles Bolden |
 | Ronald McNair |
 | Frederick D. Gregory |