Hydrogen facts for kids

Purple glow in its plasma state
|
|||||||||||||||||||||
| Hydrogen | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appearance | colorless gas | ||||||||||||||||||||
| Standard atomic weight Ar, std(H) | [1.00784, 1.00811] conventional: 1.008 | ||||||||||||||||||||
| Hydrogen in the periodic table | |||||||||||||||||||||
|
|||||||||||||||||||||
| Atomic number (Z) | 1 | ||||||||||||||||||||
| Group | group 1: hydrogen and alkali metals | ||||||||||||||||||||
| Period | period 1 | ||||||||||||||||||||
| Block | s | ||||||||||||||||||||
| Electron configuration | 1s1 | ||||||||||||||||||||
| Electrons per shell | 1 | ||||||||||||||||||||
| Physical properties | |||||||||||||||||||||
| Phase at STP | gas | ||||||||||||||||||||
| Melting point | 13.99 K (−259.16 °C, −434.49 °F) | ||||||||||||||||||||
| Boiling point | 20.271 K (−252.879 °C, −423.182 °F) | ||||||||||||||||||||
| Density (at STP) | 0.08988 g/L | ||||||||||||||||||||
| when liquid (at m.p.) | 0.07 g/cm3 (solid: 0.0763 g/cm3) | ||||||||||||||||||||
| when liquid (at b.p.) | 0.07099 g/cm3 | ||||||||||||||||||||
| Triple point | 13.8033 K, 7.041 kPa | ||||||||||||||||||||
| Critical point | 32.938 K, 1.2858 MPa | ||||||||||||||||||||
| Heat of fusion | (H2) 0.117 kJ/mol | ||||||||||||||||||||
| Heat of vaporization | (H2) 0.904 kJ/mol | ||||||||||||||||||||
| Molar heat capacity | (H2) 28.836 J/(mol·K) | ||||||||||||||||||||
Vapor pressure
|
|||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||
| Oxidation states | −1, +1 (an amphoteric oxide) | ||||||||||||||||||||
| Electronegativity | Pauling scale: 2.20 | ||||||||||||||||||||
| Ionization energies |
|
||||||||||||||||||||
| Covalent radius | 31±5 pm | ||||||||||||||||||||
| Van der Waals radius | 120 pm | ||||||||||||||||||||
| Spectral lines of hydrogen | |||||||||||||||||||||
| Other properties | |||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||
| Crystal structure | hexagonal | ||||||||||||||||||||
| Speed of sound | 1310 m/s (gas, 27 °C) | ||||||||||||||||||||
| Thermal conductivity | 0.1805 W/(m⋅K) | ||||||||||||||||||||
| Magnetic ordering | diamagnetic | ||||||||||||||||||||
| Molar magnetic susceptibility | −3.98·10−6 cm3/mol (298 K) | ||||||||||||||||||||
| CAS Number | 12385-13-6 1333-74-0 (H2) |
||||||||||||||||||||
| History | |||||||||||||||||||||
| Discovery | Henry Cavendish (1766) | ||||||||||||||||||||
| Named by | Antoine Lavoisier (1783) | ||||||||||||||||||||
| Main isotopes of hydrogen | |||||||||||||||||||||
|
|||||||||||||||||||||
Hydrogen is a super important chemical element! Its symbol is H, and it's number 1 on the periodic table. This means it's the lightest element of all!
It's also the most common element in the whole Universe, making up about 75% of everything! Most stars, like our Sun, are made mostly of hydrogen. The most common type of hydrogen atom has just one proton and one electron spinning around it.
Contents
What is Hydrogen Like?
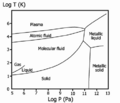
Hydrogen is a nonmetal, which is different from the other elements in its column on the periodic table (those are alkali metals). But if you could make hydrogen solid, scientists think it would act like a metal!
When hydrogen is by itself, it usually joins up with another hydrogen atom to make dihydrogen (H2). This H2 gas is very stable. At normal temperatures and pressures, hydrogen gas has no color, no smell, and no taste. It's not harmful to breathe, but it burns very easily!
How Hydrogen Burns
Hydrogen gas is flammable, meaning it can catch fire. It reacts with oxygen like this:
2 H2(g) + O2(g) → 2 H2O(l) + energy
This reaction creates water and a lot of energy! If the temperature is above 500 degrees Celsius (about 932 degrees Fahrenheit), hydrogen will catch fire on its own in the air.
Hydrogen Compounds
Even though pure hydrogen gas isn't super reactive, it loves to form compounds with many other elements. It especially likes to join with halogens, which are elements that really want to grab electrons.
Hydrogen also forms tons of compounds with carbon atoms. These are called hydrocarbons. Things like petroleum and natural gas are made of hydrocarbons. Studying hydrocarbons is a big part of organic chemistry.
Sometimes, a hydrogen atom can gain an electron and become negatively charged (H-). This is called a hydride. For example, lithium hydride (LiH) is a compound that contains a hydride.
Hydrogen and Acids
Acids that are dissolved in water usually have a lot of hydrogen ions. These are basically just free protons (hydrogen atoms that have lost their electron). The amount of these hydrogen ions helps us figure out an acid's pH level.
For example, hydrochloric acid, which is found in your stomach, can break apart into a chloride ion and a free proton. This free proton is what helps your stomach digest food by breaking it down.
Different Types of Hydrogen Atoms (Isotopes)
Hydrogen has 7 known isotopes, which are like different versions of the same atom. Two of these are stable, meaning they don't change over time:
- Protium (1H): This is the most common type, with just one proton and no neutrons.
- Deuterium (2H): This type has one proton and one neutron. It's sometimes called "heavy hydrogen."
Another important isotope is tritium (3H). It has one proton and two neutrons. Tritium is radioactive and slowly changes over time, with a half-life of about 12.33 years. Small amounts of tritium are made naturally by cosmic rays hitting the Earth's atmosphere. The other four isotopes of hydrogen are very unstable and only last for tiny fractions of a second.
Hydrogen in Nature
On Earth, pure hydrogen is usually a gas. Hydrogen is also a key part of a water molecule (H2O). It's super important because it's the fuel that powers our Sun and other stars!
Hydrogen makes up about 74% of the entire Universe. Its symbol on the Periodic Table of Elements is H.
Pure hydrogen gas is usually made of two hydrogen atoms joined together. Scientists call these diatomic molecules. Hydrogen loves to have chemical reactions when mixed with most other elements. It has no color or smell.
You won't find much pure hydrogen in the Earth's atmosphere. That's because it's so light that most of it would have floated away into space a long, long time ago. In nature, hydrogen is usually found in water.
Hydrogen is also in all living things! It's a part of the organic compounds that make up plants, animals, and people. Plus, hydrogen atoms can combine with carbon atoms to form hydrocarbons. Petroleum and other fossil fuels are made of these hydrocarbons and are often used to create energy.
Here are some cool facts about hydrogen:
- It's a gas at room temperature.
- It acts like a metal when it's solid.
- It's the lightest element in the Universe.
- It's the most common element in the Universe.
- It burns or explodes when it touches a flame.
- It glows purple when it's in a plasma state.
History of Hydrogen
Hydrogen was first separated by a scientist named Robert Boyle in 1671. Then, in 1776, Henry Cavendish figured out that it was a unique element. He also discovered that when hydrogen burns, it creates water!
Antoine Lavoisier was the one who gave hydrogen its name. He took it from Greek words: 'υδορ (pronounced /HEEW-dor/), which means "water," and gennen, which means "to generate." So, hydrogen basically means "water-former" because it makes water when it reacts with oxygen.
How We Use Hydrogen
Hydrogen is used a lot in the petroleum industry. It helps turn heavy oils into lighter, more useful ones. It's also used to make ammonia through a process called the Haber process. A small amount of hydrogen is used as fuel, for example, in rockets for spacecraft. Most of the hydrogen we use comes from a chemical reaction between natural gas and steam.
Nuclear Fusion
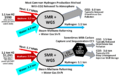
Nuclear fusion is an incredibly powerful way to create energy. It works by pushing atoms together to make helium and release a huge amount of energy. This is exactly what happens inside a star like our Sun, or in a hydrogen bomb. It takes a lot of energy to get fusion started, and scientists are still working on making it easier to do here on Earth.
One big advantage of fusion over nuclear fission (which is used in today's nuclear power stations) is that it creates much less nuclear waste. It also doesn't use dangerous and rare fuels like uranium. Did you know that more than 600 million tons of hydrogen undergo fusion every second on the Sun? That's a lot of power!
Hydrogen as a Fuel
Hydrogen can be burned to create heat for things like steam turbines or internal combustion engines. Just like other synthetic fuels, hydrogen can be made from natural fuels like coal or natural gas, or even from electricity. This means it can be a valuable part of our power grid.
Many countries, like Japan, Korea, and several in Europe, are planning to use hydrogen more in their energy systems, including fuel cell vehicles. This helps them rely less on petroleum, which is good for their economy. Another great thing about using hydrogen in a fuel cell or a hydrogen car engine is that it doesn't create pollution. It only produces water vapor and a tiny bit of nitrogen oxides.
| Periodic table | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
Images for kids
-
NGC 604, a huge cloud of glowing hydrogen gas where new stars are being born in the Triangulum Galaxy.
-
This picture shows how hydrogen can be made from methane pyrolysis.
See also
 In Spanish: Hidrógeno para niños
In Spanish: Hidrógeno para niños
 | Mary Eliza Mahoney |
 | Susie King Taylor |
 | Ida Gray |
 | Eliza Ann Grier |