Isotopes of hydrogen facts for kids
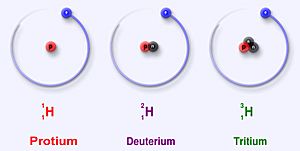
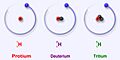
Hydrogen is a very common element, and it has different versions called isotopes. Think of isotopes like different models of the same car – they're all cars, but they have slightly different features. Hydrogen has three main isotopes that are found naturally: protium (which is the most common), deuterium, and tritium.
Protium and deuterium are stable, meaning they don't change over time. Tritium, however, is a bit different. It's radioactive, which means it slowly changes into another element. This process takes about 12 years for half of the tritium to change. Scientists have also made four other hydrogen isotopes in labs, but these are very unstable and don't last long in nature.
What makes hydrogen's main isotopes special is that they are the only ones that have their own names! These names are still used today. Sometimes, you might even see deuterium called "D" and tritium called "T". Even though the International Union of Pure and Applied Chemistry (a group that sets rules for chemistry) doesn't officially love these special symbols, people still use them a lot.
Contents
Understanding Protium (Hydrogen-1)
Protium is the most common type of hydrogen. It makes up more than 99.98% of all the hydrogen found in the universe! It's called protium because its center, called the nucleus, has only one proton.
A proton is a tiny particle with a positive charge. Protium is very light, with an atomic mass of about 1.00782504 u. Its symbol is 1H.
Scientists believe protium is a very stable isotope. This means its proton has never been seen to decay (break down) into other particles. Some modern theories in particle physics suggest that protons *might* decay, but if they do, it would happen incredibly slowly. We're talking about a half-life of 1036 years, which is an unbelievably long time! This means that for all practical purposes, protium is considered stable.
Exploring Deuterium (Hydrogen-2)
Deuterium, also known as 2H or sometimes D, is another stable isotope of hydrogen. Unlike protium, deuterium has one proton and one neutron in its nucleus. A neutron is another tiny particle found in the nucleus, but it has no electric charge.
Deuterium makes up a small part of the hydrogen on Earth, about 0.0026% to 0.0184%. You'll find more of it in sea water than in hydrogen gas. For example, sea water contains about 0.015% deuterium. Deuterium is not radioactive, so it's safe for living things.
When deuterium combines with oxygen, it forms a special kind of water called heavy water. This is different from regular water because it has deuterium instead of protium.
Discovering Tritium (Hydrogen-3)
Tritium (3H) is the most stable radioactive isotope of hydrogen. This means that out of all the radioactive forms of hydrogen, tritium is the one that lasts the longest before changing. Its nucleus has one proton and two neutrons.
Tritium decays by a process called beta minus decay. When it decays, it changes into helium-3. This process has a half-life of 12.32 years. This means that after about 12 years, half of a sample of tritium will have changed into helium-3.
Tritium forms naturally when cosmic rays (tiny, super-fast particles from space) hit the gases in Earth's upper atmosphere. Tritium and deuterium are also used together in a process called D-T nuclear fusion inside stars. This process releases a huge amount of energy, which is how stars shine!
Images for kids
See also
 In Spanish: Anexo:Isótopos de hidrógeno para niños
In Spanish: Anexo:Isótopos de hidrógeno para niños
 | Isaac Myers |
 | D. Hamilton Jackson |
 | A. Philip Randolph |