Radioactive decay facts for kids
Radioactive decay is a natural process that happens to some chemical elements. Most elements are stable, meaning their atoms stay the same forever. Even during a chemical reaction, the atoms themselves don't change.
In the late 1800s, a scientist named Henri Becquerel found that some elements have atoms that actually change over time. In 1898, Marie and Pierre Curie named this amazing process "radioactive decay." Becquerel and the Curies won the Nobel Prize in Physics in 1903 for their important discovery.
Contents
How Atoms Change: An Example
Most carbon atoms have six protons and six neutrons in their center, called the nucleus. This type of carbon is called carbon-12. Its total weight is 12 (6 protons + 6 neutrons).
But some carbon atoms have two extra neutrons, making them carbon-14. Carbon-14 acts like other carbon in chemical ways because its six protons and six electrons control its chemical properties. You can find carbon-14 in all living things, like plants and animals.
However, carbon-14 is radioactive. It changes over time through a process called beta decay and becomes nitrogen-14. The small amount of carbon-14 found in nature is harmless. Scientists use carbon-14 in archeology to figure out how old wood and other old living things are. This method is called radiocarbon dating.
Different Ways Atoms Decay
Ernest Rutherford discovered that radioactive atoms decay in different ways. He found two main types, which he called alpha decay and beta decay. Later, in 1900, Paul Villard found a third type. Rutherford named it gamma decay in 1903.
When radioactive carbon-14 changes into stable nitrogen-14, it's an example of radioactive decay. This happens when the atom gives off an electron.
Scientists later found other kinds of decay. Each type of decay produces different kinds of tiny particles. The original radioactive atom is called the "parent nucleus." The new atom it changes into is called the "daughter nucleus." The high-energy particles released by radioactive materials are known as radiation.
Sometimes, atoms decay one after another in a "decay chain." One type of atom decays into another, which then decays again, and so on. This chain continues until it forms a stable atom that doesn't decay anymore.
How Fast Atoms Decay
The speed at which radioactive atoms change is different for each element. Radioactive decay happens by chance. The time it takes for half of the atoms in a substance to decay is called its half-life.
For example, iodine (131I) has a half-life of about 8 days. This means that after 8 days, half of the iodine-131 atoms will have decayed. The half-life of plutonium can range from just 4 hours (for 243Pu) to 80 million years (for 244Pu).
Energy from Nuclear Changes
When an atom undergoes radioactive decay, its nucleus changes from a higher energy state to a lower energy state. The extra energy is released and carried away by the particles created during the decay. This energy can be released as a gamma ray (a type of light), a beta particle, or an alpha particle. In all these cases, the total number of positive and negative charges in the atom stays balanced before and after the change.
Alpha Decay
During alpha decay, the atomic nucleus releases an alpha particle. An alpha particle is made of two protons and two neutrons. When an atom loses these, it changes into a completely different element. This is because the number of protons defines the element. For example, if Americium goes through alpha decay, it changes into Neptunium. Neptunium has two fewer protons than Americium. Alpha decay usually happens in very heavy elements like uranium, thorium, plutonium, and radium.
Alpha particles cannot travel far; they can't even go through a few centimeters of air. Alpha radiation usually can't harm humans if the source is outside the body, because our skin blocks the alpha particles. However, alpha radiation can be very dangerous if it gets inside the body. This can happen if someone breathes in dust or gas that contains materials emitting alpha particles.
Beta Decay
There are two types of beta decay: beta-minus and beta-plus.
In beta-minus decay, the nucleus gives off a negatively charged electron. At the same time, one of its neutrons changes into a proton:
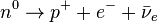 .
.
- where
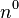 is the neutron
is the neutron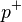 is the proton
is the proton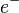 is the electron
is the electron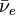 is the anti-neutrino
is the anti-neutrino
Beta-minus decay happens in places like nuclear reactors.
In beta-plus decay, the nucleus releases a positron. A positron is like an electron but with a positive charge. Also, a proton changes into a neutron:
Beta-plus decay occurs inside the sun and in some types of particle accelerators.
Gamma Decay
Gamma decay happens when a nucleus releases a high-energy packet of energy called a gamma ray. Gamma rays do not have an electrical charge, but they carry energy. Gamma rays are often released by nuclei right after other types of decay.
Gamma rays have many uses. They can be used to see through materials, to kill bacteria in food, to help find some types of diseases, and to treat some kinds of cancer. Gamma rays have the highest energy of any electromagnetic wave. Very powerful gamma ray bursts from space are the most energetic releases of energy known in the universe.
Related pages
Images for kids
-
A simulation of many identical atoms decaying. The number at the top shows how many half-lives have passed.
-
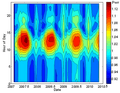
This is a gamma-ray energy spectrum of uranium ore. Gamma-rays are released by decaying nuclides, and their energy helps identify which nuclide is decaying. Here, several nuclides from the decay chain of 238U are identified: 226Ra, 214Pb, 214Bi.
See also
 In Spanish: Radiactividad para niños
In Spanish: Radiactividad para niños



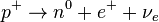 .
.
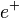 is the
is the 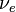 is the
is the