Ernest Rutherford facts for kids
Quick facts for kids
The Lord Rutherford of Nelson
|
|
|---|---|

Rutherford c. 1920s
|
|
| President of the Royal Society | |
| In office 1925–1930 |
|
| Preceded by | Sir Charles Scott Sherrington |
| Succeeded by | Sir Frederick Gowland Hopkins |
| Personal details | |
| Born | 30 August 1871 Brightwater, Colony of New Zealand |
| Died | 19 October 1937 (aged 66) Cambridge, England |
| Resting place | Westminster Abbey |
| Citizenship | New Zealand naturalised British subject |
| Spouse | Mary Georgina Newton (m. 1900–1937, his death) |
| Children | 1 daughter (Eileen Mary Rutherford) |
| Residences | New Zealand, United Kingdom |
| Signature | |
| Alma mater | University of New Zealand Cavendish Laboratory, University of Cambridge |
| Known for |
|
| Awards |
|
| Scientific career | |
| Fields | radioactivity, atomic physics, nuclear physics |
| Institutions |
|
| Academic advisors |
|
| Doctoral students |
|
| Other notable students |
|
| Influenced |
|
Ernest Rutherford was a brilliant scientist from New Zealand. He is often called the 'father of nuclear physics' because of his amazing discoveries about atoms. Many people consider him one of the greatest experimental scientists ever. He spent much of his career working in Canada and the United Kingdom.
Rutherford made many important discoveries. He found out about radioactive half-life, which is how long it takes for half of a radioactive substance to decay. He also discovered the element radon. He was the first to name and explain alpha and beta radiation. He did this work at McGill University in Canada.
Because of this important work, he won the Nobel Prize in Chemistry in 1908. He was the first person from Oceania to win a Nobel Prize. He was also the first to win it for work done in Canada.
In 1907, Rutherford moved to the University of Manchester in the UK. There, he and Thomas Royds proved that alpha radiation is actually helium nuclei. Rutherford's most famous work happened after he won the Nobel Prize. In 1911, he suggested that atoms have a tiny, dense center called the atomic nucleus. This idea led to the Rutherford model of the atom. He figured this out by studying how alpha particles scattered when they hit a thin piece of gold foil in the Geiger–Marsden experiment.
Later, in 1917, he performed the first artificial nuclear reaction. He bombarded nitrogen with alpha particles. This led to his discovery of a new subatomic particle. In 1920, he named this particle the proton.
Rutherford became the Director of the Cavendish Laboratory at the University of Cambridge in 1919. Under his leadership, the neutron was discovered by James Chadwick in 1932. Also, in the same year, his students John Cockcroft and Ernest Walton performed the first controlled experiment to split an atom's nucleus. Ernest Rutherford passed away in 1937 and was buried in Westminster Abbey. The chemical element rutherfordium (element 104) is named after him.
Contents
Ernest Rutherford's Life Story
Early Life and Education
Ernest Rutherford was born on August 30, 1871, in Brightwater, near Nelson, New Zealand. His father, James Rutherford, was a farmer. His mother, Martha Thompson, was a schoolteacher. When Ernest's birth was registered, his first name was accidentally spelled 'Earnest'.
He went to Havelock School and then Nelson College. He was a very good student and won a scholarship to study at Canterbury College. While there, he joined the debating society and played rugby. After getting his degrees, he spent two years doing research. During this time, he even invented a new type of radio receiver.
In 1895, Rutherford won a special scholarship. This allowed him to travel to England for more studies. He went to the Cavendish Laboratory at the University of Cambridge. He was one of the first students from outside Cambridge to do research there. He worked under the famous scientist J. J. Thomson. With Thomson's help, Rutherford was able to detect radio waves from far away. He even held a world record for detecting electromagnetic waves over a long distance.
In 1898, Thomson suggested Rutherford for a job at McGill University in Montreal, Canada. Rutherford accepted the position. This meant he could marry Mary Georgina Newton in 1900. They had one daughter named Eileen Mary. In 1907, he moved back to Britain. He became a professor of physics at the Victoria University of Manchester.
Later Years and Special Honours
Ernest Rutherford was knighted in 1914, which means he was given the title 'Sir'. During World War I, he worked on a secret project. This project aimed to find submarines using sonar. In 1919, he returned to the Cavendish Laboratory. He took over from J. J. Thomson as the director.
Under Rutherford's leadership, many important discoveries were made. James Chadwick discovered the neutron in 1932. John Cockcroft and Ernest Walton split the atom using a particle accelerator. Edward Victor Appleton showed that the ionosphere exists.
From 1925 to 1930, Rutherford was the President of the Royal Society. This is a very important scientific group. He also helped almost 1,000 university refugees from Germany. In 1931, he was given the title Baron Rutherford of Nelson. This made him a Lord, but the title ended when he died.

Rutherford had a small health problem that he didn't get fixed. It became very serious, and he became very ill. Despite an emergency operation, he died four days later in 1937. He was buried in Westminster Abbey. This is a very high honor, and he rests near other famous scientists like Isaac Newton.
Ernest Rutherford's Scientific Discoveries

At Cambridge, Rutherford worked with J. J. Thomson. They studied how X-rays affected gases. This work helped lead to the discovery of the electron in 1897. Rutherford then became interested in Henri Becquerel's work with uranium. He started to explore its radioactivity. He found two types of radiation that were different from X-rays.
Continuing his research in Canada, he named these two types of radiation. In 1899, he called them alpha rays and beta rays. He also discovered that thorium gave off a radioactive gas. This gas would make other substances radioactive. He found that it always took the same amount of time for half of a radioactive sample to decay. He called this its "half-life".
From 1900 to 1903, a young chemist named Frederick Soddy joined him. They worked together to identify the thorium gas. They found it was an inert gas and named it thoron (now known as an isotope of radon). They also found other radioactive substances.
In 1903, Rutherford and Soddy published their "Law of Radioactive Change." Before this, people thought atoms were indestructible. But Rutherford and Soddy showed that radioactive atoms actually break apart. This was a completely new and exciting idea! The Nobel Prize in Chemistry in 1908 was given to Ernest Rutherford for this groundbreaking work.
In 1903, Rutherford also studied a type of radiation discovered by Paul Villard. This radiation came from radium and could pass through things much better than alpha or beta rays. Rutherford named this third type of radiation the gamma ray. Today, all three of Rutherford's terms (alpha, beta, gamma) are still used.
In 1904, Rutherford suggested that radioactivity could explain why the Sun has shone for millions of years. Scientists like Lord Kelvin thought the Earth was much younger. This was because they didn't know of enough energy sources to keep the Sun burning for so long. Rutherford showed that radioactivity could provide that energy.
In Manchester, he kept studying alpha radiation. He worked with Hans Geiger to count alpha particles. They used special screens and chambers. By doing this, Rutherford figured out that an alpha particle had a charge of two. Later, Rutherford and Thomas Royds proved that alpha particles were actually helium nuclei.
For a long time, people thought Rutherford was the first to change one element into another. They believed he changed nitrogen into oxygen. However, later research showed that Patrick Blackett was the one who actually proved this change. Rutherford did discover the proton coming out of the nitrogen nucleus. But Blackett showed that the final product was oxygen.
The Gold Foil Experiment
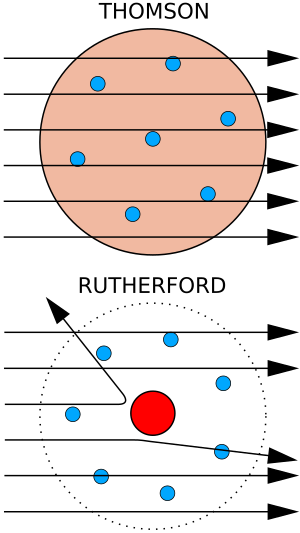
Bottom: What Rutherford observed: a few particles bounced back, showing there was a tiny, dense center. The diagram is not to scale; the nucleus is much, much smaller than the electron cloud.
Rutherford did his most famous experiment after winning the Nobel Prize. In 1909, he worked with Hans Geiger and Ernest Marsden. They did the Geiger–Marsden experiment, also known as the gold foil experiment. They shot tiny alpha particles at a very thin piece of gold foil.
Rutherford asked them to look for alpha particles that bounced back at very sharp angles. This was not expected at all based on what scientists thought about atoms then. But, surprisingly, some particles did bounce back! This showed that atoms were not just a uniform "plum pudding" of positive charge.
In 1911, Rutherford used this data to create his Rutherford model of the atom. He suggested that an atom has a very tiny, dense, positively charged center called the nucleus. Most of the atom's mass is in this nucleus. Light electrons then orbit around this nucleus.
In 1919–1920, Rutherford found that when alpha particles hit nitrogen and other light elements, a proton was ejected. He called this a "hydrogen atom" at first. This result showed him that hydrogen nuclei were part of nitrogen nuclei. He thought that the hydrogen nucleus might be a basic building block for all nuclei. In 1920, he named this new particle the proton.
In 1921, Rutherford worked with Niels Bohr. Bohr suggested that electrons move in specific orbits around the nucleus. Rutherford then thought about the existence of neutrons. He believed neutrons could help hold the nucleus together. They would balance the repelling force between the positive protons. This was a mystery because nuclei had about twice the mass that could be explained by just protons.
Rutherford famously said about his experiment: "It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you."
Rutherford's idea of neutrons was proven in 1932 by his colleague James Chadwick. Chadwick recognized neutrons when they were produced by other scientists. He later produced them himself by hitting beryllium with alpha particles. In 1935, Chadwick won the Nobel Prize in Physics for discovering the neutron.
Ernest Rutherford's Legacy

Ernest Rutherford is considered one of the greatest scientists in history.
Father of Nuclear Physics
Rutherford's research helped us understand the nuclear structure of the atom. He showed that radioactive decay is a process that happens inside the nucleus. His student, Patrick Blackett, showed how to change one element into another using natural alpha particles. Later, Rutherford's team used protons from an accelerator to create artificial nuclear reactions. Because of all this, he is known as the 'father of nuclear physics'.
Rutherford passed away before Leó Szilárd came up with the idea of controlled nuclear chain reactions. However, a speech Rutherford gave in 1933 inspired Szilárd. Rutherford talked about his students splitting lithium atoms. He realized the energy released was huge. But he also knew that the accelerators were not efficient enough to make it a practical energy source.
Things Named After Rutherford

Many things are named to honor Ernest Rutherford and his work:
- The element rutherfordium (Rf), which is element 104.
- The rutherford (Rd), an old unit for measuring radioactivity.
- The Rutherford Appleton Laboratory, a science lab in England.
- Rutherford College, Auckland, a school in New Zealand.
- Rutherford College, Kent, a college at the University of Kent in England.
- The Rutherford Medal, the top science award in New Zealand.
- The Rutherford Memorial Medal, an award for physics and chemistry research in Canada.
- The Rutherford Medal and Prize, awarded for research in nuclear physics.
- The Rutherford Memorial Lecture, a lecture series by the Royal Society.
- Rutherford Discovery Fellowships, awarded by the Royal Society of New Zealand.
- Rutherford House, a boarding house at Nelson College.
- Rutherford Hotel, the biggest hotel in Nelson, New Zealand.
- The physics and chemistry building at the University of Canterbury in New Zealand.
- Rutherford House, a main building at Victoria University of Wellington in New Zealand.
- A building at the modern Cavendish Laboratory in Cambridge.
- The Ernest Rutherford Physics Building at McGill University in Canada.
- The Coupland Building at the University of Manchester was renamed "The Rutherford Building" in 2006.
- Lord Rutherford Road, where he was born in Brightwater, New Zealand.
- Rutherford Street, a main road in Nelson, New Zealand.
- Rutherfordstraße, a street in Berlin, Germany.
- Rutherford Road in Carlsbad, California, USA.
- Rutherford Road in Vaughan, Ontario, Canada.
- Rutherford – Pickering Memorial in Havelock, New Zealand.
- Rutherford Park, a sports ground in Nelson, New Zealand.
- The Rutherford Memorial at his birthplace in Brightwater, New Zealand.
- His picture is on the New Zealand one hundred-dollar note.
- The Rutherford Foundation, a trust that supports science research in New Zealand.
- Rutherford House at Macleans College in Auckland, New Zealand.
- Rutherford House at Hillcrest High School in Hamilton, New Zealand.
- Rutherford House at Rotorua Intermediate School in Rotorua, New Zealand.
- Rutherford House at Rangiora High School.
- The crater Rutherford on the Moon.
- The crater Rutherford on the planet Mars.
- Ernest Rutherford was the subject of a play by Stuart Hoar.
- There is an engraving of a crocodile at the Mond Laboratory in Cambridge. This was Rutherford's nickname given by his friend Pyotr Kapitsa.
- The Rutherford rocket engine, developed in New Zealand by Rocket Lab.
- His image is in a stained glass window at Lindisfarne College, New Zealand.
See also
 In Spanish: Ernest Rutherford para niños
In Spanish: Ernest Rutherford para niños
- Bateman equation
- Hydrophone
- Magnetic detector
- Neutron generator
- Rutherford–Bohr model
- Rutherfordine
- The Rutherford Journal



