Bohr model facts for kids
The Bohr model is a simple way to understand how an atom works. It was created by a scientist named Niels Bohr. This model is especially good at explaining the hydrogen atom and how it gives off light, which is called its emission spectrum. It's a key idea in physics, especially in a field called quantum mechanics.
Electrons and Energy
Electron Energy Levels
The Bohr model helps us understand where electrons are found inside an atom. It says that electrons can only exist at certain energy levels. Think of these levels like steps on a ladder. An electron can be on step 1, step 2, or step 3, but never floating in between steps.
This idea means that an electron's energy is "quantized." It can only have specific amounts of energy. When an electron moves from one energy level to another, it happens instantly. It doesn't slowly travel between the levels.
How Light is Made
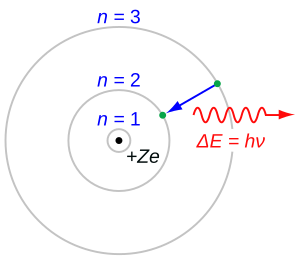
Bohr's model also explains why atoms give off light. Light is a form of energy that travels as both waves and tiny particles called photons.
When an electron in an atom jumps from a higher energy level to a lower one, it loses energy. This lost energy is then released as a photon. The energy of this photon decides what kind of light or radiation is created. For example, if an electron drops a lot of energy, it might release a photon of blue light. If it drops less energy, it might release a photon of red light.
Related pages
See also
 In Spanish: Modelo atómico de Bohr para niños
In Spanish: Modelo atómico de Bohr para niños
 | Isaac Myers |
 | D. Hamilton Jackson |
 | A. Philip Randolph |