Otto Hahn facts for kids
Quick facts for kids
Otto Hahn
|
|
|---|---|
 |
|
| Born | 8 March 1879 |
| Died | 28 July 1968 (aged 89) |
| Nationality | German |
| Alma mater | University of Marburg University of Munich |
| Known for |
|
| Spouse(s) |
Edith Junghans
(m. 1913) |
| Children | Hanno Hahn (1922–1960) |
| Awards |
|
| Scientific career | |
| Fields |
|
| Institutions |
|
| Doctoral advisor | Theodor Zincke |
| Other academic advisors |
|
| Doctoral students |
|
| Signature | |
 |
|
Otto Hahn (born March 8, 1879 – died July 28, 1968) was a German chemist. He was a pioneer in studying radioactivity and radiochemistry. People often call him the "father of nuclear chemistry" and the "father of nuclear fission".
Hahn, along with Lise Meitner, discovered radioactive versions (called isotopes) of elements like radium, thorium, protactinium, and uranium. He also found out about atomic recoil (when an atom kicks back after releasing a particle) and nuclear isomerism (when an atom has the same number of protons and neutrons but different energy). He also helped create a way to date rocks using rubidium–strontium dating. In 1938, Hahn, Lise Meitner, and Fritz Strassmann made the huge discovery of nuclear fission. This is why Hahn received the 1944 Nobel Prize in Chemistry. Nuclear fission became the basis for nuclear reactors and nuclear weapons.
Otto Hahn earned his doctorate from the University of Marburg in 1901. He then studied with famous scientists like Sir William Ramsay in London and Ernest Rutherford in Montreal. During this time, he discovered several new radioactive isotopes. In 1906, he returned to Germany. Emil Fischer gave him a small lab space at the University of Berlin. By 1912, Hahn was leading the Radioactivity Department at the new Kaiser Wilhelm Institute for Chemistry. He worked closely with Lise Meitner there, making important discoveries, including isolating the longest-lived isotope of protactinium in 1918.
During World War I, Hahn served in the army. He was part of a special unit that used chemicals. After the war, he became the head of the Kaiser Wilhelm Institute for Chemistry. From 1934 to 1938, he worked with Strassmann and Meitner. They studied what happened when neutrons hit uranium and thorium. This research led to their discovery of nuclear fission. Hahn was against the Nazi Party and their actions. Many of his colleagues, including Meitner, had to leave Germany. During World War II, he worked on a German project to study uranium. After the war, he was held by Allied forces from July 1945 to January 1946.
After the war, Hahn helped rebuild German science. He was the last president of the Kaiser Wilhelm Society and the first president of its successor, the Max Planck Society, from 1948 to 1960. In 1959, he helped start the Federation of German Scientists. This group promotes responsible science. He became one of the most respected citizens in West Germany.
Contents
- Early Life and Education
- Discovering New Elements
- Finding Mesothorium I
- Radioactive Recoil and New Institute
- Family Life
- World War I and Scientific Work
- Leading the Institute
- During the Nazi Era
- Leading the Max Planck Society
- Speaking for Social Responsibility
- Death and Legacy
- See also
- Publications in English
- Images for kids
Early Life and Education
Otto Hahn was born in Frankfurt am Main on March 8, 1879. He was the youngest son of Heinrich Hahn, a successful glass maker. His mother was Charlotte Hahn. Otto had an older half-brother and two older brothers. The family lived above his father's workshop.
When he was 15, Otto became very interested in chemistry. He did simple experiments in his family's laundry room. His father wanted him to study architecture. But Otto convinced him that he wanted to be an industrial chemist.
In 1897, Hahn began studying chemistry at the University of Marburg. He also studied mathematics, physics, and philosophy. He spent some time at the University of Munich. In 1901, he earned his doctorate in Marburg. His research was on a topic in organic chemistry. After his military service, he worked as an assistant at the University of Marburg for two years.
Discovering New Elements

Hahn wanted to work in industry. He got a job offer that required him to live in another country and improve his English. So, in 1904, Hahn went to University College London. He worked with Sir William Ramsay, who had discovered inert gases. Here, Hahn started working in radiochemistry, a very new field at the time.
In 1905, while working with radium salts, Hahn found a new substance. He called it radiothorium (thorium-228). People thought it was a new radioactive element. Later, scientists realized it was an isotope of thorium. The idea of isotopes was only named in 1913. Ramsay was excited about this discovery and announced it to the Royal Society.

Hahn published his findings in May 1905. This was the first of over 250 scientific papers he would write on radiochemistry. Ramsay suggested Hahn go to the University of Berlin. But Hahn decided to learn more about radioactivity first. He wrote to Ernest Rutherford, a leading expert in the field. Rutherford agreed to take Hahn as an assistant.
From September 1905 to mid-1906, Hahn worked with Rutherford's team at McGill University in Montreal. Some scientists doubted radiothorium existed. But Hahn proved it did. He also found other short-lived "elements," which were actually isotopes. Rutherford once said that Hahn had a "special nose for discovering new elements."
Finding Mesothorium I
In 1906, Hahn returned to Germany. Emil Fischer gave him a former woodworking shop to use as a lab. Hahn set up his equipment to measure different types of radiation. He bought radium and got thorium for free.
Within a few months, Hahn discovered mesothorium I (radium-228) and mesothorium II (actinium-228). He also found ionium (thorium-230), which is the parent substance of radium. Mesothorium I became very important. It was used in medical radiation treatments, just like radium-226, but it cost less. Hahn also found that he couldn't separate mesothorium from radium.
In 1907, Hahn became a university lecturer (Privatdozent). Most chemists at the institute didn't think Hahn's work was "real chemistry." But physicists were more open to it. At a physics meeting in September 1907, he met the Austrian physicist Lise Meitner. She was one of the first women to get a doctorate from the University of Vienna. This meeting started a 30-year partnership and a lifelong friendship.
At first, it was hard for Meitner. Women were not allowed in universities in Prussia yet. She could only work in the wood shop, which had its own entrance. She couldn't go into the rest of the institute. The next year, women were allowed in universities, and the rules changed.
Radioactive Recoil and New Institute
In 1904, Harriet Brooks had seen a radioactive recoil, but she didn't understand it fully. Hahn and Meitner were able to show and correctly explain radioactive recoil. This happens when an atom emits an alpha particle with great force. The atom then recoils, like a gun kicking back when fired. This recoil is strong enough to break chemical bonds.
In 1910, Hahn became a professor. Two years later, he became the head of the Radioactivity Department. This was at the newly built Kaiser Wilhelm Institute for Chemistry in Berlin. The new building was very helpful. Their old lab had become contaminated with radioactive materials. In the new labs, Hahn and Meitner set up strict rules. They kept chemical and physical measurements separate. They also made sure people handling radioactive substances followed safety steps.
Family Life

With a steady income, Hahn could now get married. In June 1911, he met Edith Junghans (1887–1968). She was an art student. They got engaged in November 1912 and married on March 22, 1913. After their honeymoon in Italy, they visited Vienna and Budapest.
Their only child, Hanno Hahn, was born on April 9, 1922. Hanno became a respected art historian. Sadly, in August 1960, Hanno died in a car accident in France. His wife also died in the accident. They left behind a 14-year-old son, Dietrich Hahn. In 1990, a prize for Italian art history was created in memory of Hanno and Ilse Hahn.
World War I and Scientific Work
In July 1914, just before World War I started, Hahn was called to serve in the army. He was awarded the Iron Cross for his actions. In 1915, he was asked to join a special unit led by chemist Fritz Haber. This unit developed and used chemical gases in warfare. Hahn was involved in several gas attacks on the Western and Eastern Fronts. He also helped test poisonous gases and gas masks.
Even during the war, Hahn continued his scientific work. He would return to Berlin when he could. There, he worked with Meitner in their lab.
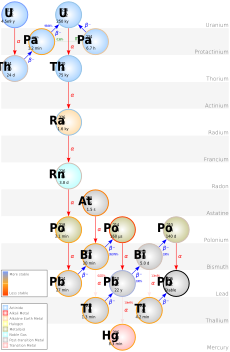
Discovering Protactinium
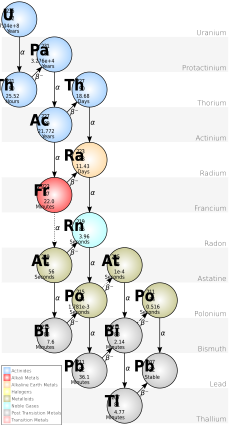
In 1913, chemists Frederick Soddy and Kasimir Fajans found that when atoms undergo radioactive decay, they change their position on the periodic table. This left a gap between thorium and uranium. Soddy predicted an unknown element there. Fajans and Oswald Helmuth Göhring soon discovered an isotope of this missing element, which they called "brevium." However, it was not the parent isotope of actinium.
Hahn and Meitner set out to find the correct parent isotope. Their work was interrupted by World War I. Meitner worked as an X-ray nurse for a while. But she returned to the Kaiser Wilhelm Institute in October 1916. Hahn was also stationed in Berlin for part of 1917. Most of their assistants were called to war. So, Hahn and Meitner did all the work themselves.
By December 1917, Meitner was able to isolate the substance. After more work, they proved it was the missing isotope. Meitner and Hahn published their findings in March 1918. Even though Fajans and Göhring found the element first, the longest-lived isotope usually gives the element its name. So, Meitner and Hahn named the element protactinium (Pa). The connection to uranium remained a mystery until uranium-235 was discovered in 1929.
Hahn and Meitner were nominated for the Nobel Prize in Chemistry many times for this discovery. In 1949, the International Union of Pure and Applied Chemistry (IUPAC) officially named the element protactinium. They confirmed Hahn and Meitner as its discoverers.
Understanding Nuclear Isomerism
After the war, Hahn looked at his earlier results from 1914. He noticed some unusual things he had overlooked. He found traces of a radioactive substance with a half-life of about 6 to 7 hours. He named this new isotope "uranium Z." In February 1921, he published his discovery.
Hahn found that uranium Z had a half-life of about 6.7 hours. He also saw that when uranium X1 decayed, it mostly became uranium X2. But about 0.25% of the time, it became uranium Z. Hahn concluded that uranium Z and uranium X2 were both the same isotope of protactinium (protactinium-234). However, they decayed at different rates.
Uranium Z was the first example of nuclear isomerism. This means that atoms can have the same number of protons and neutrons but different energy states. This discovery was very important for nuclear physics, even if its full meaning wasn't clear at the time.
Leading the Institute

In 1924, Hahn became a full member of the Prussian Academy of Sciences in Berlin. He remained head of his department. In 1928, he became the director of the Kaiser Wilhelm Institute for Chemistry. Meitner became the director of the Physical Radioactivity Division. Hahn led the Chemical Radioactivity Division.
In the early 1920s, Hahn started a new area of research called "applied radiochemistry." This field used radioactivity to study general chemical and physical questions. In 1936, his book Applied Radiochemistry was published. It contained his lectures from when he was a visiting professor at Cornell University in 1933. This book greatly influenced nuclear chemists and physicists around the world. Hahn is known as the father of nuclear chemistry.
During the Nazi Era
Fritz Strassmann came to work at the Kaiser Wilhelm Institute for Chemistry. After the Nazi Party came to power in Germany in 1933, Strassmann refused to join the Nazi Party. This meant he couldn't work in the chemical industry or get an academic position. Meitner convinced Hahn to hire Strassmann as an assistant. Soon, Strassmann was recognized as a key partner in their research.
Hahn was in the United States and Canada from February to June 1933. A new law in April 1933 banned Jewish people from universities. Meitner was from Austria, so she was not directly affected at first. Many scientists had to leave Germany. Hahn did not have to fire his own staff. But as a temporary director at another institute, he had to dismiss some staff.
Hahn worked to help his colleagues. He helped ensure some funds were given to dismissed staff to help them leave Germany. He also attended a memorial service for Fritz Haber, a Jewish scientist who had resigned in protest. University professors were not allowed to attend, so they sent their wives. Hahn, Max Planck, and Joseph Koeth attended and spoke.
Dating Rocks with Rubidium–Strontium
While Hahn was in North America, he learned about a mineral from Manitoba that contained rubidium. He had previously studied the radioactive decay of rubidium-87. He thought he could measure the age of the mineral by comparing the amount of strontium (which came from decayed rubidium) to the remaining rubidium. This method would be better than using uranium decay, because uranium decay can produce helium, which can escape and make rocks seem younger.
Strassmann and Ernst Walling extracted strontium from the mineral. They found that all of it was strontium-87, meaning it came from rubidium-87 decay. The age of the mineral was estimated to be 1,975 million years. This matched Hahn's earlier calculation for rubidium-87's half-life. Rubidium–strontium dating became a widely used method for dating rocks.
Discovering Nuclear Fission
In 1932, James Chadwick discovered the neutron. Then, Irène Curie and Frédéric Joliot found that hitting aluminum with alpha particles made it radioactive. This showed that radioactivity could be created where it wasn't before. Enrico Fermi and his team in Rome started hitting elements with neutrons.
Fermi's group bombarded uranium with neutrons. They found a complex mix of radioactive products. Fermi thought they had created new elements heavier than uranium (called transuranium elements). Meitner and Hahn decided to repeat Fermi's experiments. They wanted to see if the new products were isotopes of protactinium or transuranium elements.
From 1934 to 1938, Hahn, Meitner, and Strassmann found many radioactive products. They believed these were transuranium elements. They were the first to measure the half-life of uranium-239. They also chemically confirmed it was a uranium isotope. However, they couldn't identify the real element 93.
In May 1937, they published their findings. Hahn was confident about their chemical findings. But Meitner was becoming unsure. She thought the reactions might be from different uranium isotopes. She also considered that it might be another case of nuclear isomerism.
After Germany took over Austria in March 1938, Meitner lost her Austrian citizenship. She had to flee to Sweden. She continued to write to Hahn. In late 1938, Hahn and Strassmann found evidence of a light element, barium, in their samples. Finding barium was very puzzling. It didn't fit with their idea of creating heavier elements. It seemed impossible to turn uranium into barium by removing so many particles.
Hahn discussed these results with Niels Bohr, Lise Meitner, and Otto Robert Frisch in Copenhagen. Further experiments showed that the isotopes behaved like barium, not radium.
Meitner agreed that a "thoroughgoing breakup" seemed difficult to explain. But she noted that nuclear physics had many surprises. On December 22, 1938, Hahn sent a paper to a science journal. He reported their chemical results. He later added that some elements they thought were transuranium might actually be lighter elements. By January 1939, he was convinced that light elements were forming. He published a new version of his article, taking back his earlier claims of finding transuranium elements.
As a chemist, Hahn was careful about making a big physics claim. But Meitner and Frisch developed a theory to explain it. They called it nuclear fission. In January and February 1939, they published articles confirming their theory. In their second paper, Hahn and Strassmann used the term Uranspaltung (uranium fission). They also predicted that more neutrons would be released during fission. This opened the door to the idea of a nuclear chain reaction. This was proven by Frédéric Joliot's team in March 1939.
World War II and Aftermath
In April 1939, scientists warned the German War Ministry about the possibility of an atomic bomb. After World War II began, the German Army took control of the German nuclear weapons program. Hahn was involved in many meetings about this project. With his assistants, he cataloged about one hundred fission product isotopes. They also studied ways to separate isotopes and purify uranium.
On February 15, 1944, the Kaiser Wilhelm Institute for Chemistry was hit by a bomb. Hahn's office and many of his belongings were destroyed. The institute moved to Tailfingen in southern Germany. Hahn and his family also moved there.
Hahn tried to help colleagues who faced difficulties because they were married to Jewish women. He arranged for some to work at the institute, claiming their work was vital to the uranium project.
Being Held at Farm Hall
On April 25, 1945, Hahn was arrested by Allied forces in Tailfingen. He handed over 150 reports about his uranium research. He was taken to Hechingen and then to a château in Versailles. There, he joined other German scientists. They were later moved to Modave, Belgium, and then flown to England.
On July 3, they arrived at Farm Hall near Cambridge. Unknown to them, their conversations were secretly recorded. They were given British newspapers. Hahn was upset by reports of German territory being given to Poland and the USSR. In August 1945, the scientists learned about the atomic bombing of Hiroshima. This made the reason for their detention clear.
Hahn was glad they had not succeeded in making a bomb. The scientists wrote a report about their project. They noted that fission was discovered by Hahn and Strassmann. They were shocked again when they learned Nagasaki was destroyed by a plutonium bomb. This meant the Allies had mastered both uranium enrichment and nuclear reactor technology. On January 3, 1946, they were allowed to return to Germany. Hahn, Werner Heisenberg, and others went to Göttingen.
The Nobel Prize in Chemistry 1944
On November 16, 1945, the Royal Swedish Academy of Sciences announced that Hahn had won the 1944 Nobel Prize in Chemistry. He received it "for his discovery of the fission of heavy atomic nuclei." Hahn was still at Farm Hall. So, he learned about his award from a newspaper. His fellow scientists celebrated with speeches and songs.
Hahn had been nominated for Nobel Prizes many times before. The Nobel Committee for Chemistry reviewed the nominations. They were impressed by Hahn's work. They decided that Hahn alone should receive the chemistry prize.
Under Nazi rule, Germans were forbidden to accept Nobel Prizes. So, the award was delayed for a year. When the Academy reconsidered in September 1945, the war was over. Hahn became the only winner of the 1944 Nobel Prize for Chemistry.
Hahn was allowed to return to Germany in January 1946. But he had trouble getting permission to travel to Sweden. So, the Nobel Foundation extended the deadline. On December 10, 1946, Hahn received his Nobel Prize medal and diploma from King Gustav V. Hahn gave 10,000 krona of his prize money to Strassmann, but Strassmann refused to use it.
Leading the Max Planck Society
After the war, Max Planck was made interim president of the Kaiser Wilhelm Society. Planck asked Hahn to be the next president. Hahn was still held in England at the time. He didn't think he was a good choice, but his colleagues convinced him. He took office on April 1, 1946.
The Allies decided to dissolve the Kaiser Wilhelm Society. But the British allowed it to continue in their zone if the name was changed. Hahn and Heisenberg were very upset. They felt the name was important for science. In September 1946, a new Max Planck Society was created. Hahn became its founding president in 1948. It took over the institutes of the former Kaiser Wilhelm Society.
Hahn served as president of the Max Planck Society until 1960. He helped it regain its reputation for excellent scientific research. New institutes were founded, and old ones grew. The budget and workforce increased significantly.
Speaking for Social Responsibility
After World War II, Hahn strongly opposed using nuclear energy for military purposes. He believed it was wrong to use scientific discoveries for such destructive ends. He became a spokesperson for scientists' social responsibility.
In 1954, he wrote an article called "Cobalt 60 – Danger or Blessing for Mankind?" It was about the misuse of atomic energy. This article was widely published and broadcast. The next year, he helped create the Mainau Declaration of 1955. In this declaration, he and other Nobel Prize winners warned about the dangers of atomic weapons. They urged nations to avoid using "force as a final resort." In 1956, Hahn repeated his appeal with 52 Nobel colleagues from around the world.
Hahn also helped write the Göttingen Manifesto in 1957. In this, he and 17 leading German atomic scientists protested against arming West Germany with nuclear weapons. Hahn met with the German Chancellor, Konrad Adenauer, and other officials. The generals argued that the army needed nuclear weapons. Adenauer agreed. The German forces were then equipped with US nuclear weapons.
In November 1957, Hahn warned about the "dangers of A- and H-bomb-experiments." He said that "today war is no means of politics anymore – it will only destroy all countries in the world." His speech was broadcast internationally. In December 1957, he repeated his appeal for radio in all Warsaw Pact states.
In 1959, Hahn co-founded the Federation of German Scientists (VDW) in Berlin. This group works to ensure science is used responsibly. Its members consider the military, political, and economic effects of their research. They share their findings with the public and leaders. Until his death, Otto Hahn continued to warn about the dangers of the nuclear arms race and radioactive contamination of the planet.
Death and Legacy
Hahn gradually became weaker and died in Göttingen on July 28, 1968. His wife Edith died only two weeks later. He was buried in the Stadtfriedhof in Göttingen.
Otto Hahn received many honors and awards from around the world during his lifetime. At the end of 1999, a German news magazine asked 500 leading scientists about the most important scientists of the 20th century. Hahn was ranked third, after Albert Einstein and Max Planck. This made him the most important chemist of his time.
Besides the Nobel Prize in Chemistry (1944), Hahn received many other awards, including:
- the Emil Fischer Medal (1922)
- the Cannizaro Prize (1938)
- the Copernicus Prize (1941)
- the Max Planck Medal, with Lise Meitner (1949)
- the Goethe Medal (1949)
- the Golden Paracelsus Medal (1953)
- the Faraday Lectureship Prize (1956)
- the Grotius Medal (1956)
- Wilhelm Exner Medal (1958)
- the Helmholtz Medal (1959)
- and the Harnack medal in Gold (1959).
Hahn became the honorary president of the Max Planck Society in 1962. He was also elected a Foreign Member of the Royal Society (1957). He received honorary memberships from many foreign academies and scientific societies. He was an honorary citizen of Frankfurt am Main, Göttingen, and Berlin. He was made an Officer of the Ordre National de la Légion d'Honneur of France (1959). He also received the Grand Cross First Class of the Order of Merit of the Federal Republic of Germany (1959).
In 1966, US President Lyndon B. Johnson and the United States Atomic Energy Commission (AEC) gave Hahn, Lise Meitner, and Fritz Strassmann the Enrico Fermi Award. Hahn received honorary doctorates from several universities, including the University of Göttingen, Technische Universität Darmstadt, and the University of Cambridge.
Many things are named after Hahn:
- the NS Otto Hahn, a nuclear-powered civilian ship (1964)
- a crater on the Moon
- the asteroid 19126 Ottohahn
- the Otto Hahn Prize
- the Otto Hahn Medal and the Otto Hahn Award
- and the Otto Hahn Peace Medal in Gold (1988).
Some chemists suggested naming element 105 hahnium in his honor. But in 1997, the IUPAC named it dubnium. In 1992, a German team discovered element 108 and suggested naming it hassium. Despite the tradition of letting the discoverer suggest a name, a committee recommended hahnium. After protests, the name hassium (Hs) was adopted internationally in 1997.
See also
 In Spanish: Otto Hahn para niños
In Spanish: Otto Hahn para niños
- List of peace activists
Publications in English
- Hahn, Otto (1936). Applied Radiochemistry. Ithaca, New York: Cornell University Press.
- Hahn, Otto (1950). New Atoms: Progress and Some Memories. New York-Amsterdam-London-Brussels: Elsevier Inc..
- Hahn, Otto (1966). Otto Hahn: A Scientific Autobiography. New York: Charles Scribner's Sons.
- Hahn, Otto (1970). My Life. New York: Herder and Herder.
Images for kids
-
Physicists and chemists in Berlin in 1920. Front row, left to right: Hertha Sponer, Albert Einstein, Ingrid Franck, James Franck, Lise Meitner, Fritz Haber, and Otto Hahn. Back row, left to right: Walter Grotrian, Wilhelm Westphal, Otto von Baeyer, Peter Pringsheim and Gustav Hertz