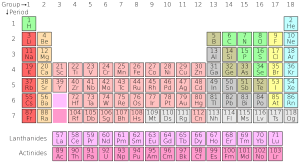Periodic table facts for kids
The periodic table of chemical elements is a special chart that lists all the known chemical elements. Think of it like an organized list of all the basic building blocks of everything around us! As of 2022, scientists have found and confirmed 118 different elements.
In this amazing table, elements are placed in order based on their atomic number. This number starts with 1 for hydrogen, which is the lightest element. An element's atomic number tells you how many protons are in the center (nucleus) of one of its atoms.
The periodic table is arranged into rows called periods and columns called groups.
- A row across the table is a period. Periods are numbered from 1 to 8.
- Period 1 has only two elements: hydrogen and helium.
- Periods 2 and 3 each have 8 elements. Other periods are longer.
- Elements in the same period have atomic numbers that go up one by one.
A column down the table is called a group. There are 18 groups in the standard periodic table, numbered from 1 to 18. Elements in the same group often have similar ways their electrons are arranged. This means they usually behave in similar chemical ways. For example, group 18 elements are called noble gases. They are all gases and usually don't mix with other atoms.
For a long time, groups were numbered using Roman numerals (like I, II, III). But in 1990, a group of scientists called the International Union of Pure and Applied Chemistry (IUPAC) decided to use the simpler Arabic numerals (1, 2, 3) for group numbers.
Scientists called chemists use the periodic table to find patterns and connections between elements. There are three main types of elements in the table: metals, metalloids (which are like a mix), and nonmetals. For example, elements at the bottom and far left of the table are the most metallic. Elements at the top right are the least metallic. This means cesium is much more metallic than helium. Many other cool patterns exist too!
The periodic table was created by a Russian chemist named Dmitry Ivanovich Mendeleyev (1834-1907). To honor him, element 101 was named mendelevium.
Contents
What the Periodic Table Looks Like
The most common way to show the periodic table is below. It helps us see how elements are organized.
| Group → | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||
| Period ↓ | ||||||||||||||||||||
| 1 | 1 H |
2 He |
||||||||||||||||||
| 2 | 3 Li |
4 Be |
5 B |
6 C |
7 N |
8 O |
9 F |
10 Ne |
||||||||||||
| 3 | 11 Na |
12 Mg |
13 Al |
14 Si |
15 P |
16 S |
17 Cl |
18 Ar |
||||||||||||
| 4 | 19 K |
20 Ca |
21 Sc |
22 Ti |
23 V |
24 Cr |
25 Mn |
26 Fe |
27 Co |
28 Ni |
29 Cu |
30 Zn |
31 Ga |
32 Ge |
33 As |
34 Se |
35 Br |
36 Kr |
||
| 5 | 37 Rb |
38 Sr |
39 Y |
40 Zr |
41 Nb |
42 Mo |
43 Tc |
44 Ru |
45 Rh |
46 Pd |
47 Ag |
48 Cd |
49 In |
50 Sn |
51 Sb |
52 Te |
53 I |
54 Xe |
||
| 6 | 55 Cs |
56 Ba |
Lanthanides | 72 Hf |
73 Ta |
74 W |
75 Re |
76 Os |
77 Ir |
78 Pt |
79 Au |
80 Hg |
81 Tl |
82 Pb |
83 Bi |
84 Po |
85 At |
86 Rn |
||
| 7 | 87 Fr |
88 Ra |
*Actinides |
104 Rf |
105 Db |
106 Sg |
107 Bh |
108 Hs |
109 Mt |
110 Ds |
111 Rg |
112 Cn |
113 Nh |
114 Fl |
115 Mc |
116 Lv |
117 Ts |
118 Og |
||
| 8 | 119 Uue |
120 Ubn |
**Superactinides |
158 Upo |
159 Upo |
160 Uhn |
161 Uhu |
162 Uhb |
163 Uht |
164 Uhq |
165 Uhp |
166 Uhh |
167 Uhs |
168 Uho |
169 Uhe |
170 Usn |
171 Usu |
172 Usb |
||
| 9 | 173 Ust |
174 Usq |
||||||||||||||||||
| * Lanthanide Series | 57 La |
58 Ce |
59 Pr |
60 Nd |
61 Pm |
62 Sm |
63 Eu |
64 Gd |
65 Tb |
66 Dy |
67 Ho |
68 Er |
69 Tm |
70 Yb |
71 Lu |
|||||
| ** Actinide Series | 89 Ac |
90 Th |
91 Pa |
92 U |
93 Np |
94 Pu |
95 Am |
96 Cm |
97 Bk |
98 Cf |
99 Es |
100 Fm |
101 Md |
102 No |
103 Lr |
|||||
| *** Superactinide Series | 143 Uqt |
144 Uqq |
145 Uqp |
146 Uqh |
147 Uqs |
148 Uqo |
149 Uqe |
150 Upn |
151 Upu |
152 Upb |
153 Upt |
154 Upq |
155 Upp |
156 Uph |
157 Ups |
|||||
| *** Superactinide Series | 121 Ubu |
122 Ubb |
123 Ubt |
124 Ubq |
125 Ubp |
126 Ubh |
127 Ubs |
128 Ubo |
129 Ube |
130 Utn |
131 Utu |
132 Utb |
133 Utt |
134 Utq |
135 Utp |
136 Uth |
137 Uts |
138 Uto |
139 Ute |
140 Uqn |
141 Uqu |
142 Uqb |
Understanding the Colors and Borders
The colors and borders around each element box in the table tell us more about them!
- Chemical Series of the Periodic Table
-
Superactinides
- State at normal conditions
The color of the number (the atomic number) above the element symbol shows what state the element is in at normal room temperature and pressure:
- Blue numbers mean the element is a gas.
- Green numbers mean the element is a liquid.
- Black numbers mean the element is a solid.
The type of border around an element's box tells us about its stability:
-
Elements with solid borders have stable forms (called primordial elements).
-
Elements with dashed borders only have naturally occurring forms that are radioactive.
-
Elements with dotted borders do not occur naturally; they are made by scientists (Synthetic Elements).
-
Elements without borders are too radioactive to have been found yet.
The History of the Periodic Table
The idea of organizing the elements is not new. As early as 1817, a scientist named Johann Wolfgang Döbereiner noticed that he could group some elements into threes, which he called triads. The elements in each triad had similar properties.
The big breakthrough came in 1869 from a Russian chemist named Dmitri Mendeleev. He wrote down the properties of each known element on a card and began arranging them. He noticed that if he put them in order of their atomic weight, a pattern appeared.
Mendeleev's genius was that he wasn't afraid to leave gaps in his table. He predicted that these gaps were for elements that had not yet been discovered. He even described the properties of three of these missing elements. Within 15 years, all three elements—gallium, scandium, and germanium—were discovered, and their properties matched Mendeleev's predictions almost perfectly. This made the scientific world realize how powerful his table was.
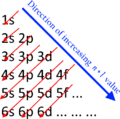
Later, scientists like Henry Moseley discovered that it was the atomic number (the number of protons), not the atomic weight, that was the key to the order of the elements. This discovery cleared up a few problems in Mendeleev's table and led to the modern periodic table we use today.
The Future of the Periodic Table
The seventh row of the periodic table was completed in 2016 with the naming of four new elements: nihonium (113), moscovium (115), tennessine (117), and oganesson (118). So what's next?
Scientists are now trying to create elements for an eighth row, starting with element 119. These are called superheavy elements. Creating them is incredibly difficult. Scientists have to smash smaller atoms together in powerful machines called particle accelerators, hoping they will stick together for a fraction of a second.
Researchers are also searching for an "island of stability". This is a theory that suggests there might be certain superheavy elements that are much more stable than the ones created so far. If such elements could be made, they might have amazing new properties that we can't even imagine yet. The quest to expand the periodic table is an exciting frontier of science.
Other Ways to See the Elements
While the table above is the most common, scientists have thought of other cool ways to show the periodic table:
-
This spiral arrangement by Theodor Benfey places elements around hydrogen. Their atomic weight helps decide their spot.
-
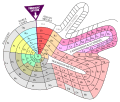
Dmitry Ivanovich Mendeleyev also tried a flower-like design. The Actinides and Lanthanides are shown as loops next to the main part.
More Ways to Explore Elements
There are many different versions and lists of elements you can find:
- A vertical table can be easier to read on computer screens.
- The big table shows the basics plus the full names of elements.
- The huge table includes full names and atomic masses.
- You can also find tables showing Electron configurations.
- There are tables that highlight metals and non-metals.
- A list of elements includes their name, symbol, atomic number, atomic mass, group, and period. You can sort this list by any of these details!
- You can also find a list of elements by symbol.
- Or lists of elements by their boiling point, melting point, or density.
Related Pages
Images for kids
See also
 In Spanish: Tabla periódica de los elementos para niños
In Spanish: Tabla periódica de los elementos para niños
 | Aaron Henry |
 | T. R. M. Howard |
 | Jesse Jackson |