Periodic table (big) facts for kids
The periodic table is like a giant map of all the known chemical elements in the universe! It helps scientists and students understand how different elements are related to each other and how they behave. Each element is a basic substance that cannot be broken down into simpler ones. Think of them as the building blocks of everything around us, from the air we breathe to the stars in the sky.
This amazing chart organizes elements based on their atomic number (which is the number of protons in an atom's nucleus). It also groups them by their chemical properties, meaning how they react with other elements.
| * Lanthanides | lanthanum 57 La |
cerium 58 Ce |
praseodymium 59 Pr |
neodymium 60 Nd |
promethium 61 Pm |
samarium 62 Sm |
europium 63 Eu |
gadolinium 64 Gd |
terbium 65 Tb |
dysprosium 66 Dy |
holmium 67 Ho |
erbium 68 Er |
thulium 69 Tm |
ytterbium 70 Yb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ** Actinides | actinium 89 Ac |
thorium 90 Th |
protactinium 91 Pa |
uranium 92 U |
neptunium 93 Np |
plutonium 94 Pu |
americium 95 Am |
curium 96 Cm |
berkelium 97 Bk |
californium 98 Cf |
einsteinium 99 Es |
fermium 100 Fm |
mendelevium 101 Md |
nobelium 102 No |
| Alkali metals | Alkaline earths | Lanthanide | Actinides | Transition metals |
| Poor metals | Metalloids | Nonmetals | Halogens | Noble gases |
Contents
How Elements Are Organized
The periodic table is arranged in a very specific way. It has rows called periods and columns called groups. This arrangement helps us predict how elements will behave.
Groups: Columns of Elements
The vertical columns on the periodic table are called groups. There are 18 groups in total. Elements in the same group often have similar chemical properties. This means they react in similar ways with other elements. For example, elements in Group 1 (like lithium and sodium) are all very reactive metals.
Periods: Rows of Elements
The horizontal rows on the periodic table are called periods. There are 7 periods. As you move from left to right across a period, the atomic number of the elements increases by one. This means each element has one more proton than the one before it. Elements in the same period do not have similar chemical properties in the same way as groups.
Types of Elements
Elements on the periodic table can be broadly divided into three main types: metals, nonmetals, and metalloids. Each type has different properties.
Metals
Most elements on the periodic table are metals. They are usually shiny, can conduct electricity and heat well, and can be hammered into shapes or drawn into wires. Examples include iron, copper, and gold.
Nonmetals
Nonmetals are found on the right side of the periodic table. They are usually not shiny, do not conduct electricity or heat well, and are often brittle (break easily) or are gases at room temperature. Examples include oxygen, nitrogen, and sulfur.
Metalloids
Metalloids are elements that have properties of both metals and nonmetals. They are found along the zigzag line between metals and nonmetals on the table. Silicon and arsenic are examples of metalloids. They can sometimes conduct electricity, but not as well as metals.
Who Created the Periodic Table?
The periodic table we use today is the result of many years of work by different scientists.
Dmitri Mendeleev
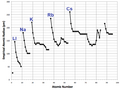
The first widely accepted periodic table was created by a Russian chemist named Dmitri Mendeleev in 1869. He arranged the elements by their atomic weight and noticed patterns in their properties. He even left gaps for elements that had not yet been discovered, and he correctly predicted their properties!
Henry Moseley
Later, in 1913, a British physicist named Henry Moseley improved Mendeleev's table. He discovered that elements should be arranged by their atomic number (the number of protons), not their atomic weight. This solved some problems in Mendeleev's original table and made the patterns even clearer.
Glenn T. Seaborg
In the mid-20th century, an American chemist named Glenn T. Seaborg made important contributions. He discovered many new elements, especially the actinides and transuranic elements. He also suggested a change to the layout of the periodic table, placing the actinide series below the main table, which is how we see it today.
Images for kids
See also
 In Spanish: Tabla periódica de los elementos para niños
In Spanish: Tabla periódica de los elementos para niños
 | Stephanie Wilson |
 | Charles Bolden |
 | Ronald McNair |
 | Frederick D. Gregory |