Krypton facts for kids
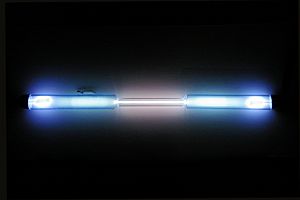
A krypton-filled discharge tube glowing white
|
|||||||||||||||
| Krypton | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈkrɪptɒn/ |
||||||||||||||
| Appearance | colorless gas, exhibiting a whitish glow in an electric field | ||||||||||||||
| Standard atomic weight Ar, std(Kr) | 83.798(2) | ||||||||||||||
| Krypton in the periodic table | |||||||||||||||
|
|||||||||||||||
| Atomic number (Z) | 36 | ||||||||||||||
| Group | group 18 (noble gases) | ||||||||||||||
| Period | period 4 | ||||||||||||||
| Block | p | ||||||||||||||
| Electron configuration | [Ar] 3d10 4s2 4p6 | ||||||||||||||
| Electrons per shell | 2, 8, 18, 8 | ||||||||||||||
| Physical properties | |||||||||||||||
| Phase at STP | gas | ||||||||||||||
| Melting point | 115.78 K (−157.37 °C, −251.27 °F) | ||||||||||||||
| Boiling point | 119.93 K (−153.415 °C, −244.147 °F) | ||||||||||||||
| Density (at STP) | 3.749 g/L | ||||||||||||||
| when liquid (at b.p.) | 2.413 g/cm3 | ||||||||||||||
| Triple point | 115.775 K, 73.53 kPa | ||||||||||||||
| Critical point | 209.48 K, 5.525 MPa | ||||||||||||||
| Heat of fusion | 1.64 kJ/mol | ||||||||||||||
| Heat of vaporization | 9.08 kJ/mol | ||||||||||||||
| Molar heat capacity | 20.95 J/(mol·K) | ||||||||||||||
Vapor pressure
|
|||||||||||||||
| Atomic properties | |||||||||||||||
| Oxidation states | 0, +1, +2 (rarely more than 0; oxide is unknown) | ||||||||||||||
| Electronegativity | Pauling scale: 3.00 | ||||||||||||||
| Ionization energies |
|
||||||||||||||
| Covalent radius | 116±4 pm | ||||||||||||||
| Van der Waals radius | 202 pm | ||||||||||||||
| Spectral lines of krypton | |||||||||||||||
| Other properties | |||||||||||||||
| Natural occurrence | primordial | ||||||||||||||
| Crystal structure | face-centered cubic (fcc) | ||||||||||||||
| Speed of sound | (gas, 20 °C) 221 m·s−1 (liquid) 1120 m/s |
||||||||||||||
| Thermal conductivity | 9.43×10−3 W/(m⋅K) | ||||||||||||||
| Magnetic ordering | diamagnetic | ||||||||||||||
| Molar magnetic susceptibility | −28.8×10−6 cm3/mol (298 K) | ||||||||||||||
| CAS Number | 7439-90-9 | ||||||||||||||
| History | |||||||||||||||
| Naming | from Greek κρυπτός, 'hidden' | ||||||||||||||
| Discovery and first isolation | William Ramsay and Morris Travers (1898) | ||||||||||||||
|
|||||||||||||||
Krypton is a fascinating chemical element. Its name comes from a Greek word meaning "the hidden one." Krypton has the symbol Kr and atomic number 36. It is a noble gas, which means it's a gas that doesn't usually react with other elements. You can't see or smell krypton because it's colorless and odorless.
Krypton is found in tiny amounts in the air around us. It's often used with other rare gases in special fluorescent lamps to create light. Like other noble gases, krypton is important in lighting and photography. The light from krypton has many different colors, called spectral lines.
Krypton gas can be used in powerful lasers. These lasers are used for many things, from light shows to scientific research. For a time, from 1960 to 1983, the official way to define a metre (a unit of length) was based on the specific color of light from a special type of krypton. This shows how stable and precise krypton's light is!
Contents
Discovering Krypton

Krypton was discovered in Britain in 1898. Two clever chemists, William Ramsay from Scotland and Morris Travers from England, found it. They discovered krypton while studying what was left after almost all parts of liquid air had evaporated. Just a few weeks later, they found another noble gas, Neon, using a similar method.
William Ramsay received the Nobel Prize in Chemistry in 1904. He was honored for discovering a group of these special noble gases, including krypton.
In 1960, scientists used krypton to define the metre. They said one meter was equal to 1,650,763.73 wavelengths of a specific light color from krypton-86. This was a very precise way to measure length. This definition was used until October 1983. Then, scientists changed the definition of the meter to be based on how far light travels in a vacuum in a tiny fraction of a second.
How Krypton Behaves
Krypton creates several bright colors of light when it's excited. The strongest colors are green and yellow. Solid krypton is white and has a common crystal structure that looks like a cube. This is true for most noble gases.
Different Forms: Krypton Isotopes
Elements can have different versions called isotopes. These isotopes have the same number of protons but different numbers of neutrons. Naturally, krypton in Earth's atmosphere has five stable isotopes. There's also one isotope, 78Kr, that lasts so long it's considered stable.
Scientists know about many other unstable isotopes of krypton. One of these, 81Kr, is made when cosmic rays hit 80Kr. This isotope is radioactive and has a half-life of 230,000 years. This means it takes 230,000 years for half of it to change into something else. 81Kr is useful for dating very old groundwater, helping scientists understand water sources from 50,000 to 800,000 years ago.
Another isotope, 85Kr, is also radioactive with a half-life of about 10.76 years. It is created during certain human activities involving nuclear energy. Scientists can use 85Kr in the atmosphere to detect specific industrial activities. For example, in the early 2000s, it helped identify facilities that were processing nuclear materials.
Krypton's Chemical Side
Krypton is known for being very unreactive. This means it doesn't easily combine with other elements to form new substances. For a long time, scientists thought noble gases couldn't form any compounds at all.
However, in the 1960s, scientists learned how to make some noble gases react. They successfully created a compound called krypton difluoride (KrF2) by making krypton react with fluorine under very special conditions.
Krypton fluoride is also used in a special type of laser. In these lasers, krypton gas reacts with fluorine gas to create a temporary compound. This compound then releases energy as light, which is used in the laser.
Scientists have even found compounds where krypton is bonded to atoms other than fluorine, like oxygen and nitrogen. They have also created special krypton hydride crystals under very high pressures.
Where is Krypton Found?
Krypton is present in the air at a very low concentration, about one ppm. This means for every million particles of air, only one is krypton. Scientists can get krypton from liquid air using a process called fractional distillation.
The amount of krypton in space is harder to measure. However, early measurements suggest that krypton is also found in space.
Amazing Uses of Krypton
Krypton's ability to produce a whitish light when ionized makes it useful in photography. It's used in some camera flashes for high-speed photos. Krypton gas can also be mixed with mercury to create bright greenish-blue light in luminous signs.
Krypton is added to argon in some energy-efficient fluorescent lamps. This helps reduce how much power the lamps use. It's also used in regular light bulbs (incandescent lamps) to help the filament last longer and burn brighter.
The white light from krypton is sometimes used for artistic effects in "neon" tubes. Krypton lasers can produce powerful red light, which is great for laser light shows.
In scientific research, a krypton fluoride laser is important for studying nuclear fusion energy. This laser can create a very uniform beam of light.
Liquid krypton is used in experimental particle physics to build special detectors called calorimeters. These detectors help scientists study tiny particles. For example, a large detector at CERN used about 27 tons of liquid krypton.
Krypton also has medical uses. A special isotope, Krypton-83, is used in magnetic resonance imaging (MRI) to create images of airways in the lungs. This helps doctors see the surfaces inside the lungs. Another isotope, krypton-81m, is used in nuclear medicine for lung scans. Patients inhale it, and a special camera takes pictures of their lungs.
Krypton is sometimes used as an insulating gas between window panes to improve energy efficiency. Even in space, krypton has a role! SpaceX uses krypton as a fuel for the electric propulsion systems on their Starlink satellites.
Staying Safe with Krypton
Krypton is not poisonous, but it is an asphyxiant. This means that if there's too much krypton in the air, it can reduce the amount of oxygen you breathe. Breathing air with too little oxygen can make you feel dizzy or even pass out.
Krypton can also make you feel sleepy or dizzy, similar to how you might feel if you were deep-sea diving. If someone were to breathe air that is 50% krypton, it would feel like breathing air at four times the normal atmospheric pressure. This effect is like scuba diving about 30 meters (100 feet) deep. It's important to be careful around krypton gas to ensure there's always enough oxygen in the air.
Images for kids
See also
 In Spanish: Kriptón para niños
In Spanish: Kriptón para niños