Thermal conductivity facts for kids
Thermal conductivity is a scientific term that describes how well a material can let heat pass through it. Think of it like a material's ability to conduct or move heat from a hotter place to a colder place. Some materials are really good at moving heat, while others are not very good at all.
Now, let's dive deeper into the fascinating world of how heat travels through different stuff around us!
Contents
The Science of Heat Movement
Heat is a form of energy, and it naturally wants to move from warmer areas to cooler areas. This movement happens in a few ways, but one important way is called conduction. Conduction is how heat travels through a material itself, without the material moving from one place to another (like water flowing or air blowing).
Imagine you hold one end of a metal spoon in a hot cup of tea. After a little while, the other end of the spoon, which isn't even touching the tea, starts to feel warm. That's heat moving through the metal spoon by conduction! The heat from the tea makes the tiny particles (atoms and molecules) in the spoon vibrate faster. These vibrating particles bump into their neighbors, making them vibrate faster too, and this chain reaction passes the heat along the spoon.
Thermal conductivity is a way to measure how quickly and easily this process happens in a specific material.
Defining Thermal Conductivity
Scientists use a number, often shown with symbols like k, λ, or κ, to represent a material's thermal conductivity.
High thermal conductivity means heat moves through the material very easily and quickly. Materials like metals (copper, silver, aluminum) have high thermal conductivity. Low thermal conductivity means heat moves through the material slowly and with difficulty. Materials like plastic, wood, glass, and air have low thermal conductivity. These are often called insulators because they resist the flow of heat.
The unit used to measure thermal conductivity in the standard scientific system (called SI units) is watts per meter-kelvin (W/(m·K)). This unit tells you how much heat energy (measured in watts) flows through a certain thickness (one meter) of a material for every degree difference in temperature (measured in kelvin, which is a temperature scale like Celsius or Fahrenheit).
Why is Thermal Conductivity Important?
Understanding thermal conductivity is super important in many parts of our lives and in technology:
- Cooking: Pots and pans are made of metals with high thermal conductivity so heat from the stove quickly and evenly spreads to the food. Handles are often made of materials with low thermal conductivity (like plastic or wood) so you don't burn your hand.
- Building Homes: Walls, roofs, and windows are designed using materials with low thermal conductivity (insulators) to keep heat inside during the winter and outside during the summer. This helps save energy used for heating and cooling.
- Electronics: Computer chips and other electronic parts get hot when they work. Materials with high thermal conductivity are used in "heat sinks" to quickly pull heat away from these parts and prevent them from overheating.
- Clothing: Winter clothes are made from materials that trap air (a good insulator) to keep your body heat from escaping.
How Heat Moves Inside Materials
At the tiny, tiny level of atoms and molecules, heat conduction happens because of:
- Vibrations: Atoms and molecules in a material are always vibrating. When one part of the material gets hotter, its particles vibrate more vigorously. These strong vibrations are passed along to neighboring particles, transferring energy (heat). This is the main way heat moves through non-metals like wood or plastic.
- Free Electrons: In metals, there are also tiny particles called electrons that can move around freely. These free electrons are really good at carrying energy, including heat energy, from one place to another very quickly. This is why metals are such good heat conductors.
Materials that are good electrical conductors (like metals) are often also good thermal conductors because the same free electrons help move both electricity and heat. However, this isn't always true! Diamond, for example, is a great thermal conductor (it moves heat very well) but doesn't conduct electricity easily. This is because heat moves through diamond mainly by vibrations of its tightly packed atoms, not by free electrons.
Measuring Thermal Conductivity
Scientists and engineers need to measure thermal conductivity accurately. There are different ways to do this, but they generally involve:
- Heating one side of a material sample.
- Keeping the other side at a cooler temperature.
- Measuring how much heat flows through the material and the temperature difference across it.
By knowing the amount of heat flow, the temperature difference, and the size and shape of the material sample, they can calculate the thermal conductivity. Some methods wait until the temperature is stable (steady-state), while others measure how the temperature changes over time as heat moves through (transient methods).
Measuring the thermal conductivity of liquids and gases can be trickier than solids because heat can also move by convection (where the fluid itself moves) and radiation (heat moving as waves, like from the sun). Special care must be taken to separate out just the heat moving by conduction.
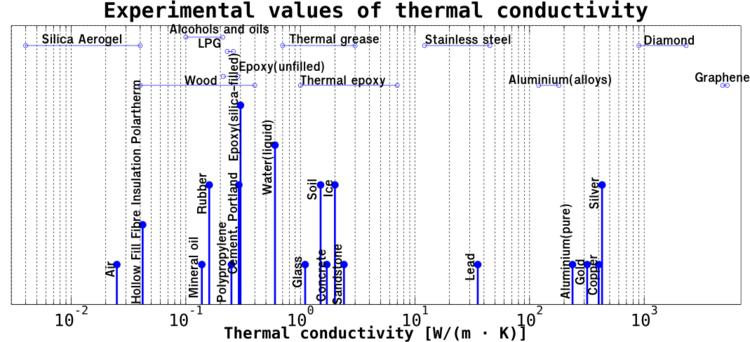
Examples of Thermal Conductivity Values
Different materials have vastly different thermal conductivity values. Here are a few examples (these are approximate values and can change slightly depending on temperature and other factors):
- Diamond: Very high (around 1000 - 2200 W/(m·K)) - one of the best natural heat conductors!
- Silver: Very high (around 429 W/(m·K))
- Copper: Very high (around 400 W/(m·K)) - commonly used in cookware and electronics.
- Aluminum: High (around 205 W/(m·K)) - also used in cookware and electronics.
- Iron: High (around 80 W/(m·K))
- Glass: Low (around 1 W/(m·K)) - used in windows, which provide some insulation.
- Water (liquid): Low (around 0.6 W/(m·K))
- Wood: Low (around 0.04 - 0.4 W/(m·K) depending on the type and direction) - used in building for insulation.
- Air: Very low (around 0.025 W/(m·K)) - this is why materials that trap air (like wool or foam) are good insulators.
Notice how metals have much higher values than materials like wood or air. This shows how much better they are at conducting heat.
What Can Affect Thermal Conductivity?
Several things can influence how well a material conducts heat:
- Temperature: For most materials, thermal conductivity changes as the temperature changes. For pure metals, it often stays pretty constant or decreases slightly as temperature increases, but for non-metals, it might increase or stay constant at higher temperatures. At very low temperatures, the behavior can be quite different.
- Chemical Phase: When a material changes from a solid to a liquid or a gas, its thermal conductivity changes a lot. For example, ice conducts heat better than liquid water. Gases are generally much poorer conductors than liquids or solids because the molecules are much farther apart.
- Anisotropy: Some materials conduct heat better in one direction than another. Wood is a good example – it conducts heat better along the grain than across it. This happens in materials where the internal structure is different in different directions.
- Density and Structure (especially in gases and porous materials): For gases and materials with lots of tiny holes (like foam or wool), how tightly packed the molecules are and the size of the gaps can affect conductivity. Trapping air in small pockets is a common way to create good insulators.
- Purity: For some very pure materials, like crystals, even tiny amounts of impurities or different versions of the atoms (isotopes) can affect how well they conduct heat, especially at low temperatures.
History
People have known for a long time that different materials conduct heat differently. Back in 1780, a scientist named Jan Ingenhousz did a simple but clever experiment to compare the thermal conductivity of different metals.
He took wires made of different metals (gold, silver, copper, steel, iron, tin, and lead) that were all the same size. He coated them in wax and then dipped one end of all the wires into hot oil. He watched how far the wax melted along each wire. The farther the wax melted, the better the metal was at conducting heat away from the hot oil.
His experiment showed that silver was the best conductor among those metals, followed by copper, then gold, and so on. This simple test helped scientists understand that materials have different abilities to move heat.
See also
 In Spanish: Conductividad térmica para niños
In Spanish: Conductividad térmica para niños
 | Emma Amos |
 | Edward Mitchell Bannister |
 | Larry D. Alexander |
 | Ernie Barnes |


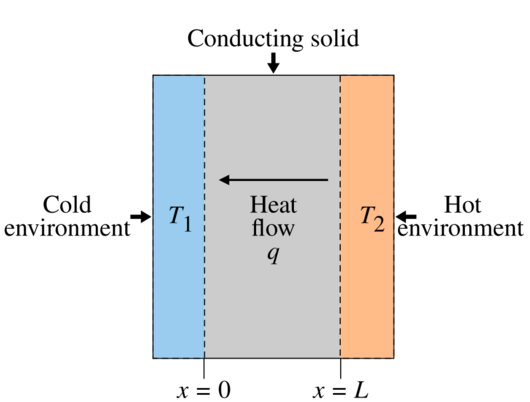
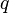 across a temperature difference.
across a temperature difference.