Diamond facts for kids
Quick facts for kids Diamond |
|
|---|---|

A scattering of round-brilliant cut diamonds shows off the many reflecting facets.
|
|
| General | |
| Category | minerals |
| Formula (repeating unit) |
C |
| Strunz classification | 01.CB.10a |
| Identification | |
| Formula mass | 12.01 g/mol |
| Color | Typically yellow, brown or gray to colorless. Less often blue, green, black, translucent white, pink, violet, orange, purple and red. |
| Crystal habit | Octahedral |
A diamond is a super hard and shiny mineral. It's made of carbon atoms, just like the lead in your pencil! The word "diamond" comes from an ancient Greek word, "adámas," which means "unbreakable."
Diamonds are the hardest natural material on Earth. Because they are so hard, many important industries use diamonds as tools. They are great for cutting and polishing other tough materials.
Most diamonds are clear, but some come in beautiful colors. You can find yellow, red, blue, green, and pink diamonds. These colorful ones are often called "fancies."
Big diamonds are very rare and cost a lot of money. Only about 20% of all diamonds found are good enough for jewellery. The other 80% are lower quality. These are called industrial diamonds. They are used to make things like drill bits and diamond saws. Even if a diamond isn't pretty enough for jewelry, it's still valuable because it's so hard.
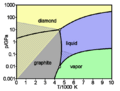
Diamonds are also interesting because they don't conduct electricity well. They are good electrical insulators. But they are very good at conducting heat! On the Mohs scale of mineral hardness, diamonds score a 10. This is the highest score possible.
Contents
How Diamonds Are Made
There are two main types of diamonds: natural and synthetic (man-made). The Earth makes natural diamonds, and people make synthetic ones.
Natural diamonds are the hardest natural substance we know. They are made of pure carbon. This is the same chemical element found in graphite (pencil lead) and coal. But diamonds are very hard and form in a crystal shape. It's a common myth that diamonds come from coal, but this isn't true.
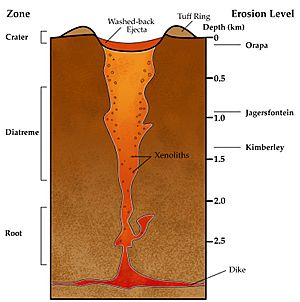
Most natural diamonds are very old. They formed between 1 billion and 3.5 billion years ago. They grew deep inside the Earth, about 150 to 250 kilometers (93 to 155 miles) down in the Earth's mantle. Some even came from as deep as 800 kilometers (500 miles)! Under very high pressure and temperature, carbon turned into diamonds. Much later, about tens to hundreds of millions of years ago, volcanoes carried these diamonds to the surface. They were found in igneous rocks called kimberlites and lamproites.
Small synthetic diamonds are made for things like abrasives (materials used for grinding or polishing). Large synthetic diamonds are usually more expensive to make than to find and dig up. So, people don't often make very large ones.
Where to Find Diamonds
People often find diamonds in places where volcanoes were active a long time ago. Sometimes, tiny diamonds are found where a meteorite has hit the Earth. You might even find diamonds on the ground's surface in some areas. But in places like South Africa, people have to dig deep into a diamond mine to get them. Diamonds were first discovered in India.
How Diamonds Are Graded
Diamonds are used as beautiful gemstones for adornment and as tools for cutting hard materials. Experts use different ways to value diamonds depending on their use.
In the 20th century, experts created a way to grade diamonds. This system is based on four important features, often called the four Cs:
- Carat: This is the diamond's weight. One carat is equal to 0.2 grams.
- Cut: This describes how well the diamond has been shaped and polished. A good cut makes a diamond sparkle.
- Color: This refers to how close the diamond is to being colorless (white). For "fancy" colored diamonds, it describes how strong their color is.
- Clarity: This means how free the diamond is from tiny marks or flaws inside it.
A very large diamond with no flaws is called a paragon.
Trading Diamonds Around the World
For many years, a company called De Beers controlled most of the diamond trade. They owned many rich diamond mines in Africa. However, in the late 1980s and early 1990s, new diamond mines opened in Canada and Australia. De Beers couldn't control these new sources. Also, when the USSR broke apart in 1991, many cheaper Russian diamonds entered the market. This made it harder for De Beers to control prices.
De Beers still runs many diamond mines in Africa. But now, their mines produce only about one-third of the world's diamonds.
Shaping Diamonds
When diamonds are first mined, they are called "rough diamonds." To turn them into sparkling gems, they go through a process called "cutting." Even though diamonds are super hard, they can also be brittle. This means they can break if hit in the right spot. So, cutting a diamond is a very careful job. It needs skill, scientific knowledge, special tools, and lots of experience.
The goal of cutting is to create a beautiful jewel with many flat surfaces called "facets." The angles between these facets are very important. They help the diamond sparkle and reflect light in the best way. The number and size of the facets also affect how much the final diamond weighs.
Diamonds can be cut into many different shapes. Some classic shapes include round, pear, marquise, and oval. There are also special shapes created by certain companies.
Protecting Diamonds from Theft
Sometimes, large amounts of diamonds are stolen. For example, in 2013, robbers stole diamonds worth about US$50 million from Brussels Airport. They broke through a fence and took the gems from a plane. The gang was later arrested, and some of the stolen diamonds and cash were found.
It can be hard to identify stolen diamonds. Rough diamonds might look different depending on where they came from. Diamonds found in rivers or on beaches often have smoother surfaces than those dug from mines. But once diamonds are cut and polished, it's much harder to tell where they came from.
The Kimberley Process was created to help stop the trade of "conflict diamonds." These are rough diamonds used to fund violence. Before rough diamonds are sent to other countries, the government of the country they came from must certify them. This helps ensure they are not conflict diamonds. However, this process does not apply to diamonds sold within a country.
Sometimes, diamonds are marked with tiny laser etchings that you can't see with your eye. This method was developed by a company called Lazare Kaplan. However, any mark on a diamond can usually be removed.
Images for kids
-
Brown diamonds at the National Museum of Natural History in Washington, D.C.
-
The most famous colored diamond, the Hope Diamond
-
Eclogite with centimeter-size garnet crystals
-
A scalpel with synthetic diamond blade
-
Close-up photograph of an angle grinder blade with tiny diamonds shown embedded in the metal
-
A diamond knife blade used for cutting ultrathin sections (typically 70 to 350 nm) for transmission electron microscopy
-
Siberia's Udachnaya diamond mine
See also
 In Spanish: Diamante para niños
In Spanish: Diamante para niños