Chemical formula facts for kids
A chemical formula is like a secret code that chemists use to describe a molecule. It tells you exactly what kinds of atoms are in the molecule and how many of each kind there are. Sometimes, it even shows how these atoms are connected or arranged in space.
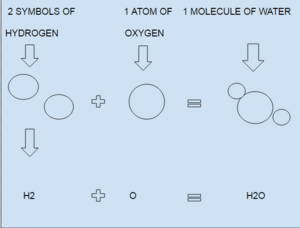
Each letter in a chemical formula stands for a different chemical element. For example, 'H' is for hydrogen and 'O' is for oxygen. The small number written below and to the right of a letter is called a subscript. This number tells you how many atoms of that element are in the molecule.
Let's look at some examples:
- Hydrogen peroxide has the formula H2O2. This means it has 2 hydrogen atoms and 2 oxygen atoms.
- Methane has the formula CH4. This tells us it has 1 carbon atom (we don't write '1' if there's only one atom) and 4 hydrogen atoms.
- The sugar molecule glucose has the formula C6H12O6. This means it has 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms.
Chemists use these formulas in chemical equations to show what happens during chemical reactions. It's a clear and simple way to communicate about chemicals.
The system for writing chemical formulas was created by a Swedish chemist named Jöns Jacob Berzelius in the 1800s.
How to Understand Chemical Formulas
Chemical formulas are a quick way to see the atoms in a substance. They use symbols from the periodic table to show each element. The periodic table is a chart that lists all the known elements. If you forget what a symbol means, you can always check the periodic table.
The small subscript numbers are very important. They tell you the exact number of atoms for each element in the formula. For instance, in H2O (water), the '2' next to 'H' means there are two hydrogen atoms. Since there's no subscript next to 'O', it means there's just one oxygen atom.
Related pages
Images for kids
See also
 In Spanish: Fórmula química para niños
In Spanish: Fórmula química para niños
 | Leon Lynch |
 | Milton P. Webster |
 | Ferdinand Smith |